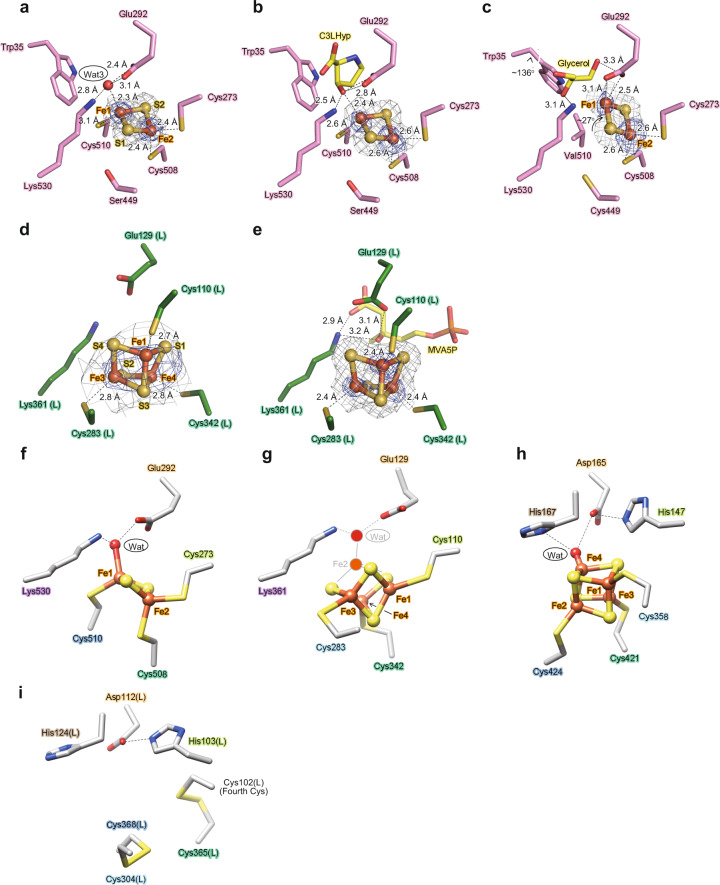
Fig. 4. Analysis of the [Fe-S] cluster in AcnX.
The observed [2Fe-2S] cluster of AtAcnX in the apo-form (a) and in complex with C3LHyp (b) of the wild-type enzyme, and in the apo-form of the S449C/C510V mutant (c). The observed [3Fe-4S] cluster of TkAcnX in the apo-form (d) and in complex with MVA5P (e). 2mFo–DFc electron density maps for the [Fe-S] cluster, contoured at 1.5 σ, are shown as a gray mesh. Anomalous difference Fourier maps, contoured at the 5.0 σ (a), 3.0 σ (b), 4.0 σ (c), 3.0 σ (d), and 9.0 σ levels (e), indicate peaks for Fe atoms, and are shown as a blue mesh. Superposition of [Fe-S] clusters of AtAcnX (f), TkAcnX (g), mAcn (h; 6ACN), and IPMI (i; 4KP2) within its active sites, ligated to protein cysteines by Fe-S bonds. Amino-acid residues located at equivalent (or close) positions are indicated in the same color and correspond to Fig. 2. The hypothetically activated [4Fe-4S] cluster of TkAcnX, the Fe2 atom, and a water molecule (gray) appeared to be coordinated to the [3Fe-4S] cluster.