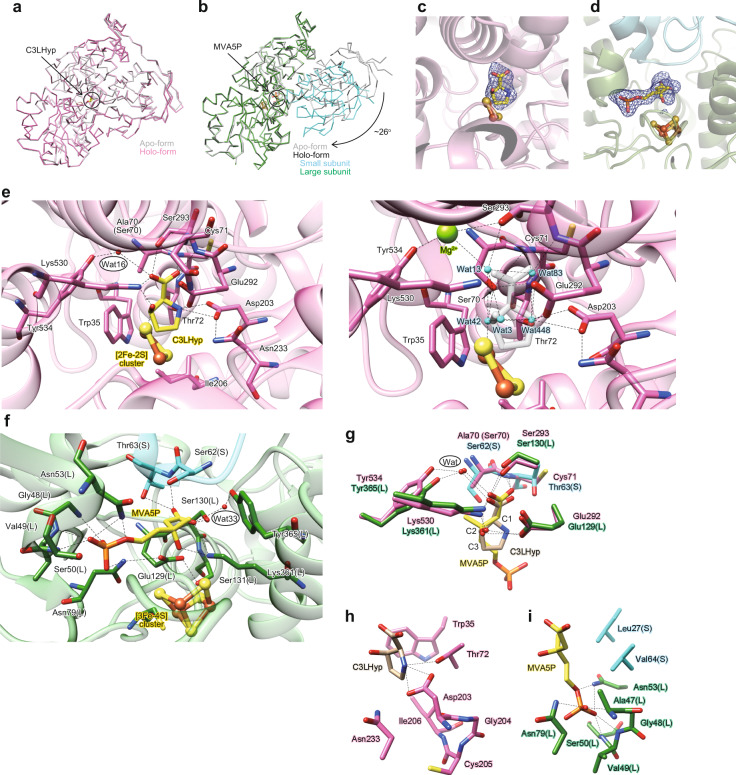
Fig. 6. Analysis of substrate-binding sites in AcnX.
A Cα trace of AtAcnX (a) and TkAcnX (b) showing similarities between the apo- and holo-forms. Bound C3LHyp and MVA5P are represented as a ball-and-stick model. In TkAcnX, the holo-form showed a more closed conformation than the apo-form by rigid-body subunit movement (~26°). Electron density maps of bound C3LHyp (c) and MVA5P (d). Simulated annealing mFo−DFc difference Fourier maps were calculated by omitting each molecule, and are shown as blue meshes countered at the 3.0 σ level. These angles were similar to those in e and f, respectively. Substrate recognition sites of AtAcnX (e) and TkAcnX (f). Active site residues are represented as stick models. The right panel of AtAcnX is the active site of the apo-form, in which C3LHyp (gray stick model) in the holo-form is superimposed. Five water molecules and one magnesium ion are represented as cyan and light-green balls, respectively. In TkAcnX, light-blue and green residues are derived from the small (S) and large subunits (L), respectively. The superposition of active sites that recognize common (g) and different structural backbones (h, i) between C3LHyp and MVA5P.