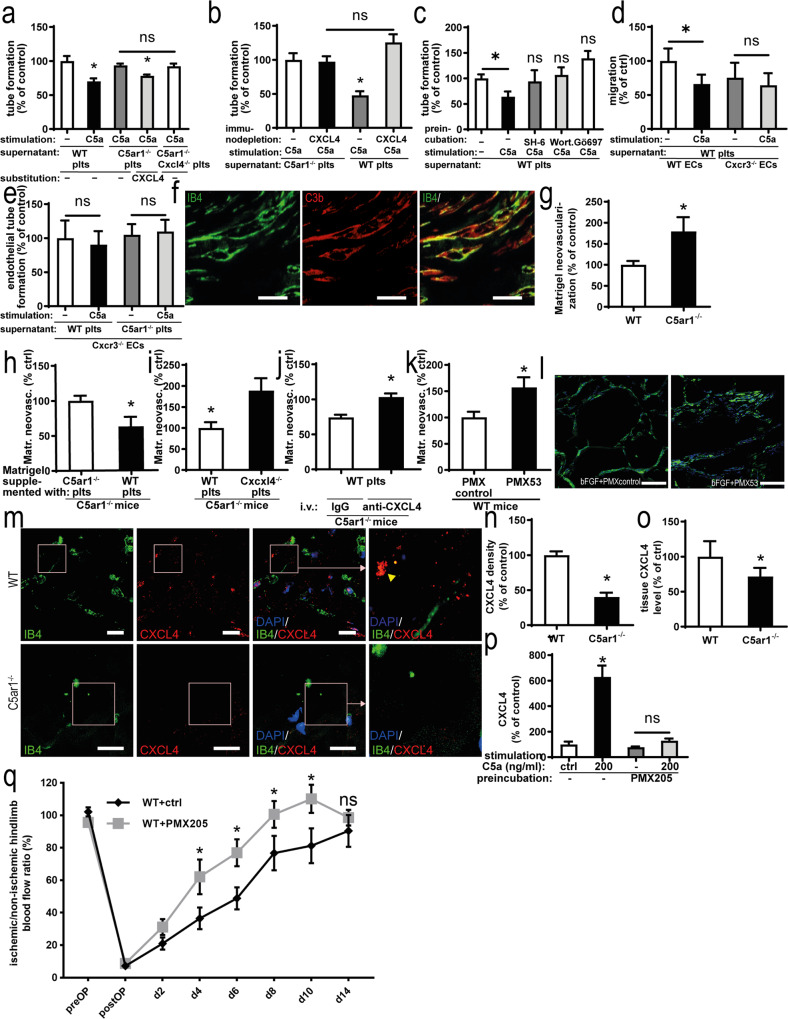
Fig. 7. Platelet C5aR1-induced inhibition of neovascularization is dependent on the secretion of CXCL4.
a MHEC-5T were coincubated with the C5a-stimulated supernatant of platelets isolated from WT, C5ar1−/−, and C5ar1−/−Cxcl4−/− mice. Furthermore, the supernatant of C5ar1−/− platelets was supplemented with CXL4 (2 µg/ml). C5a-stimulated WT platelet supernatant inhibited endothelial tube formation, which was not detectable in C5ar1−/− and C5ar1−/−Cxcl4−/− platelets. Reconstitution with CXCL4 in the C5ar1−/− group led to a similar level of tube formation as in the WT group. Data are displayed as the mean ± SEM (n = 5 independent experiments). The total tube length after treatment with vehicle-stimulated platelet supernatant represents 100%. *p < 0.05. b CXCL4 was immunodepleted from C5a-stimulated WT as well as C5ar1−/− platelet supernatant, and the impact of this supernatant on endothelial tube formation was assessed. Data are shown as the mean ± SEM (n = 5 independent experiments). The total tube length in the group with control-depleted supernatant from C5a-stimulated C5ar1−/− platelets represents 100%. *p < 0.05. c Freshly isolated washed murine WT platelets were preincubated with vehicle control or kinase inhibitors SH-6, Wortmannin, and Gö976 before stimulation with C5a. The supernatant was then coincubated with MHEC-5T and endothelial tube formation was quantified. Preincubation of platelets with SH-6, Wortmannin, and Gö976 led to a level of tube formation not significantly different from vehicle control-stimulated WT platelet supernatant. Data are shown as the mean ± SEM (n = 4–8 independent experiments). The total tube length in the group with control-preincubated vehicle control-stimulated WT platelet supernatant represents 100%. *p < 0.05. d Primary murine lung endothelial cells were isolated from Cxcr3−/− mice (for endothelial cell quality control refer to Supplementary Fig. 3). After coincubation with C5a-stimulated platelet supernatant, WT endothelial cells displayed decreased endothelial migration in the scratched wound assay. Cxcr3−/− endothelial cells exhibited a significantly smaller decrease in migration compared with WT cells. Data are displayed as the mean ± SEM (n = 6 independent experiments). Migration in the WT endothelial cell group treated with vehicle-stimulated WT platelet supernatant subtracted represents 100%. *p < 0.05. e Similarly, the decrease in endothelial tube formation was significantly smaller in Cxcr3−/− endothelial cells following coincubation with C5a-stimulated platelet supernatant compared with control supernatant and comparable to the level C5ar1−/− platelet supernatant stimulated with C5a. Data are shown as the mean ± SEM (n = 5 independent experiments). The total tube length in the group treated with vehicle-stimulated WT platelet supernatant represents 100%. *p < 0.05. f Similar to the hindlimb ischemia model (Fig. 1a), complement activation (C3b, red) colocalized with vascular structures (IB4, green) within the Matrigel plug 7 days after implantation. ×400 magnification. Scale bars represent 100 µm. Displayed is a representative image of four independent experiments. g To study the paracrine effect of platelets after C5a stimulation in vivo, we used the Matrigel model to analyze vascularization after the addition of platelets and soluble mediators. Matrigel was supplemented with bFGF and injected into WT or C5ar1−/− mice. Quantification of neovascularization after 7 days yielded a significantly higher level of growth factor-induced angiogenesis in C5ar1−/− animals than in WT controls. Data are shown as the mean ± SEM (n = 6–8 Matrigels per group). The neovascularization area fraction within Matrigels from WT mice represents 100%. *p < 0.05. h Freshly isolated WT or C5ar1−/− platelets were resuspended in Matrigel, the Matrigel was implanted into C5ar1−/− animals, and vascularization was assessed after 7 days. Data are presented as the mean ± SEM (n = 7–8 Matrigels per group). The neovascularization area fraction in Matrigels from C5ar1−/− mice supplemented with C5ar1−/− platelets represents 100% expressed as the area fraction of nuclear staining. *p < 0.05. i WT platelets or Cxcl4−/− platelets were coinjected with Matrigel into C5ar1−/− animals. Injection of Cxcl4−/− platelets or vehicle control yielded similar levels of growth factor-induced vessel formation. However, injection of WT platelets significantly reversed the phenotype of increased neovascularization in C5ar1−/− mice. Data are shown as the mean ± SEM (n = 6 Matrigels per group). The neovascularization area fraction in Matrigels from C5ar1−/− mice re-transfused with vehicle control represents the 100% value expressed as the area fraction of nuclear staining. *p < 0.05, WT platelets versus Cxcl4−/− platelets. j WT platelets were coinjected with Matrigel into C5ar1−/− mice, and animals were treated systemically with an anti-CXCL4 antibody or IgG control. While the animals that received WT platelets and IgG control showed a reduced level of growth factor-induced angiogenesis, the animals that received WT platelets and anti-CXCL4 antibody did not exhibit significantly different vessel formation levels compared with control animals that did not receive platelet retransfusion. Data are shown as the mean ± SEM (n = 7–9 Matrigels per group). The neovascularization area fraction in Matrigels from WT mice without platelet injection and vehicle control treatment represents 100% expressed as the area fraction of nuclear staining. *p < 0.001, anti-CXCL4 antibody versus IgG control. k Matrigel was supplemented with bFGF and additionally PMX53 or PMX control and injected into WT mice. Quantification of neovascularization after 7 days yielded a significantly higher level of growth factor-induced angiogenesis in the PMX53 group. Data are shown as the mean ± SEM (n = 7 Matrigels per group). The neovascularization area fraction within Matrigels from WT mice supplemented with PMX control represents 100%. *p < 0.05. l Representative immunofluorescent stainings of Matrigel plug sections at ×400 magnification shows Matrigel neovascularization (IB4 in green, nuclei in blue) is increased after supplementation of Matrigel with PMX53. Scale bars represent 100 µm. Image is representative of at least four analyzed plugs. m Ischemic hindlimb muscle tissue from WT or C5ar1−/− mice was stained for the presence of CXCL4 deposition (red) 1 week after induction of ischemia. IB4 staining (green) depicts vascular structures, DAPI (blue) nuclei. ×630 magnification, scale bars represent 2 µm. n Quantification of CXCL4 abundance by measuring the area fraction of CXCL4-positive staining in whole-muscle sections acquired by tile scanning at ×400 magnification. Data are shown as the mean ± SEM (n = 10 whole-muscle sections per group) and are displayed as the percentage of control. The area fraction of CXCL4 staining in hindlimb muscle sections from WT mice represents 100%. *p < 0.01. o Ischemic muscle tissue from the hindlimb ischemia experiments was homogenized, and samples of equal protein content were probed for CXCL4 concentration using ELISA. Homogenates from C5ar1−/− mice yielded significantly lower CXCL4 levels compared with those from WT mice. Data are shown as the mean ± SEM (n = 6–8 muscles processed). The CXCL4 concentration in WT muscle homogenates measured by ELISA represents 100%. *p < 0.05. p Freshly isolated washed murine WT platelets were isolated and preincubated with PMX205 or vehicle control. C5a-induced CXCL4 secretion was quantified by ELISA. PMX205 inhibited C5a-induced CXCL4 secretion. Data are shown as the mean ± SEM (n = 5–25 independent experiments). The CXCL4 concentration in the supernatant of WT platelets stimulated with vehicle control represents 100%. *p < 0.05. q WT mice treated with the C5aR1 inhibitor PMX205 or control were subjected to hindlimb ischemia and analyzed after 2 weeks. We observed increased revascularization in PMX205-treated animals. Data are shown as the mean ± SEM (n = 7 animals per group) and are displayed as the percentage of the perfusion in the contralateral control limb. *p < 0.05 Two-sided Student’s t test in g–k, n, o. One-way ANOVA with Bonferroni’s post hoc test in a–e, p. Two-way ANOVA with Bonferroni’s post hoc test in q.