Abstract
Background
FOLFIRINOX has shown promising results in locally advanced (LAPA) or borderline resectable (BRPA) pancreatic adenocarcinoma. We report here a cohort of patients treated with this regimen from the AGEO group.
Methods
This is a retrospective multicentre study. We included all consecutive patients with non-pre-treated LAPA or BRPA treated with FOLFIRINOX.
Results
We included 330 patients (57.9% male, 65.4% <65 years, 96.4% PS <2). Disease was classified as BRPA in 31.1% or LAPA in 68.9%. Objective response rate with FOLFIRINOX was 29.5% and stable disease 51%. Subsequent CRT was performed in 46.4% of patients and 23.9% had curative intent surgery. Resection rates were 42.1% for BRPA and 15.5% for LAPA. Main G3/4 toxicities were fatigue (15%), neutropenia (12%) and neuropathy (G2/3 35%). After a median follow-up of 26.7 months, median OS (mOS) and PFS were 21.4 and 12.4 months, respectively. For patients treated by FOLFIRINOX alone, or FOLFIRINOX followed by CRT, or FOLFIRINOX + /− CRT + surgery, mOS was 16.8 months, 21.8 months and not reached, respectively (p < 0.0001).
Conclusions
FOLFIRINOX for LAPA and BRPA seems to be effective with a manageable toxicity profile. These promising results in “real-life” patients now have to be confirmed in a Phase 3 randomised trial.
Subject terms: Pancreatic cancer, Pancreatic cancer
Background
Pancreatic adenocarcinoma (PAC) incidence has increased constantly over the past decades.1,2 It is currently the fourth leading cause of cancer death worldwide. At the time of diagnosis, 35% of PACs are considered locally advanced, and only 15% of patients have a resectable disease.3 Tumours with arterial or venous vessel involvement are considered locally advanced or borderline resectable PAC (LAPA and BRPA, respectively), depending on the type of vascular involvement.4 In summary, according to the National Comprehensive Cancer Network (NCCN) guidelines, BRPA is defined by contact ≥180° with venous vessels (superior mesenteric and portal veins) or <180° but irregular or associated with a venous occlusion that can be treated surgically or an arterial contact <180°.5,6 LAPA is defined by a venous occlusion inaccessible to reconstruction and/or arterial contact ≥180° and/or contact with the first jejunal superior mesenteric artery branch and/or aortic involvement. The current standard of care for those patients is not well defined, even though surgery followed by adjuvant chemotherapy is frequently proposed in many countries for BRPAs.7 Still, in those situations, induction treatment based on chemotherapy or chemoradiotherapy (CRT) is designed to shrink the tumour to achieve R0 resection. Nevertheless, the proportion of secondary resection is highly heterogeneous according to the studies, ranging from 5 to 50% in the current literature.8 In addition, median overall survival (OS) remains low, ranging from 6 to 24 months according to the possibility of secondary surgery.8–10 Recently, results of the PREOPANC Phase 3 trial indicated a significant improvement of OS with induction CRT vs immediate surgery in patients with BRPA but not in those with LAPA.11 Altogether, the best induction treatment for both BRPA and LAPA is still debated, and several clinical trials are ongoing.
In the past decade, FOLFIRINOX (5-fluorouracil (5FU), oxaliplatin and irinotecan) has become one of the first-line standard treatment for patients in good physical condition suffering from metastatic PAC.12 Moreover, in resectable PAC, adjuvant modified FOLFIRINOX also showed recently improved OS and disease-free survival (DFS) compared to gemcitabine alone.13 In LAPA and BRPA, the only data available concerning the use of FOLFIRINOX are mostly from retrospective studies with low numbers of patients.10,14–17
We previously published in 2015 within the AGEO (Association des Gastro-Entérologues Oncologues), a gastrointestinal (GI) oncology French network, a series of 77 patients with LAPA and BRPA treated with FOLFIRINOX with encouraging results, subsequently confirmed in a meta-analysis with other series.8,14 Here we present an update of this AGEO cohort (with >300 patients) aiming to re-assess the efficacy and tolerability of FOLFIRINOX and potential subsequent local treatment (CRT or surgery) for BRPA or LAPA. We also explored factors associated with OS.
Methods
Patients
All consecutive patients with LAPA and BRPA treated with the induction FOLFIRINOX regimen between February 2010 and December 2018 in 14 French centres were enrolled in the study. Inclusion criteria were: histologically or cytologically proven LAPA or BRPA, age >18 years, Eastern Cooperative Oncology Group performance status (ECOG PS) <3 and no metastatic lesion at baseline thoraco-abdomino-pelvic computed tomographic (CT) scan. LAPA or BRPA was defined by each centre at a multidisciplinary team (MDT) meeting at diagnosis according to the NCCN guidelines.6 Previous chemotherapy or RT or surgery for PAC and unconfirmed or doubtful cytology were a non-inclusion criterion. No informed consent was needed for this observational study, as stated by the French ethics committee consulted prior to the beginning of the work.
Treatment
FOLFIRINOX was administered as follows: oxaliplatin (85 mg/m2), leucovorin (400 mg/m2), irinotecan (180 mg/m2), and a continuous infusion (2400 mg/m2) of 5FU every 2 weeks. The administration of a 5FU bolus and dose reduction were decided by each investigator. Primary prophylaxis of neutropenia using granulocyte colony-stimulating factor (G-CSF) was initiated at the physician’s discretion. FOLFIRINOX was administered every 2 weeks until disease progression, unacceptable toxicity, consolidation treatment with CRT and/or surgery. As recommended by the French national guidelines, follow up was realised with CT scans and CA 19–9 measurement performed every 8 weeks.
Each patient’s file was discussed at an MDT meeting every 2–3 months (after 4–6 courses of induction FOLFIRINOX) in each centre. At least one senior expert radiologist, one radiotherapist and a pancreatic surgeon reviewed CT scans during these MDT meetings. For each patient, the decision regarding additional chemotherapy, CRT and/or secondary resection was based on clinical, biological and radiological data as per the local multi meeting including an expert surgeon for pancreatic cancer surgery. For the patients who underwent surgery, R0 resection was defined as resection margins >1 mm.
Statistical analysis
Median (interquartile and range) values and proportions (percentage) were provided for the description of continuous and categorical variables, respectively. Progression-free survival (PFS) was defined as the time between FOLFIRINOX start and radiological local or distant progression according to Response Evaluation Criteria In Solid Tumours (RECIST) 1.1 criteria or death, whichever occurred first. In the subgroup of patients who had secondary R0/R1 resection, DFS was defined as the time between secondary resection and local or distant relapse or death, whichever occurred first. OS was defined as the time between FOLFIRINOX start and death from any cause. Patients known to be alive were censored for PFS, DFS and OS at the date of their last follow-up. PFS, DFS and OS were estimated using the Kaplan–Meier method and described using median or rate at specific time points with their 95% confidence interval (CI). Follow-up was calculated using a reverse Kaplan–Meier estimation.
Radiological tumour response was evaluated according to RECIST 1.1 criteria on CT scan. Objective response was defined as complete and partial response; and disease control was defined as complete, partial response or stable disease. Factors associated with OS and PFS were first assessed with univariate Cox proportional hazard models. Parameters with p values of <0.10 in univariate analysis or clinically relevant variables were entered into the multivariable Cox regression model. Correlations between variables were verified before construction of the multivariate models, in order to deal with potential co-linearity.
All analyses were performed using the R software version 2.15.2 (R Development Core Team, Vienna, Austria; http://www.r-project.org). p Values of <0.05 were considered statistically significant, and all tests were two sided. The cut-off date for analysis was March 1, 2020.
Results
Patient characteristics
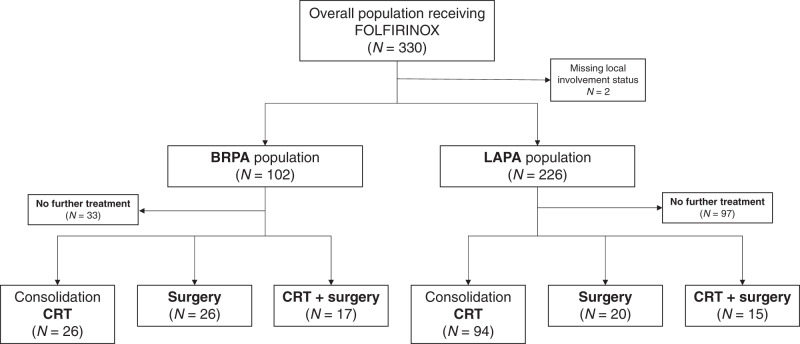
During the study period, 330 patients were enrolled, including 226 (68.9%) with LAPA and 102 (31.1%) with BRPA (2 patients were not evaluable for local involvement). Patient characteristics are summarised in Table 1. Patients were mainly men (57.9%), aged ≤65 years (65.4%), with good PS (ECOG PS 0/1 in 96.4%). Patients with BRPA had smaller tumour size, and BRPA tumours were more frequently located in the pancreatic head as compared with LAPA. The study flowchart is displayed as Fig. 1.
Table 1.
Patient and tumour characteristics.
| N (%) | Whole population N = 330 | BRPA population N = 102 | LAPA population N = 226 | p | |
|---|---|---|---|---|---|
| Sex | Female | 139 (42.1) | 37 (36.3) | 100 (44.3) | 0.17 |
| Male | 191 (57.9) | 65 (63.7) | 126 (55.7) | ||
| Age | Year, median (range) | 62.0 (56.0–67.0) | 62.0 (58.0–67.0) | 62.5 (56.0–67.0) | 0.73 |
| ≤65 | 216 (65.4) | 67 (65.7) | 147 (65.0) | 0.91 | |
| >65 | 114 (34.6) | 35 (34.3) | 79 (35.0) | ||
| Performance status | 0 | 124 (37.7) | 35 (34.3) | 89 (39.6) | 0.58 |
| 1 | 193 (58.7) | 64 (62.8) | 127 (56.4) | ||
| 2 | 12 (3.6) | 3 (2.9) | 9 (4.0) | ||
| Missing | 1 | 0 | 1 | ||
| Tumour location | Head | 233 (70.6) | 80 (78.4) | 152 (67.3) | 0.04 |
| Body | 77 (23.3) | 16 (15.7) | 61 (27.0) | ||
| Tail | 15 (4.5) | 6 (5.9) | 8 (3.5) | ||
| Isthmus | 5 (1.6) | 0 | 5 (2.2) | ||
| Tumour size (mm) | Median (range) | 37 (28–43) | 30 (25–40) | 37 (30–44) | <0.001 |
| Missing | 19 | 2 | 17 | ||
| Biliary stent | Yes | 147 (45.2) | 49 (48.0) | 97 (43.9) | 0.49 |
| No | 178 (54.8) | 53 (52.0) | 124 (56.1) | ||
| Missing | 5 | 0 | 5 | ||
| Pre-treatment CA 19-9 (IU/mL) | Median (range) | 266 (39–1200) | 152 (33–1162) | 284 (47–1227) | 0.86 |
| Missing | 36 | 9 | 27 | ||
| Number of FOLFIRINOX cycles | Median (range) | 7 (5–11) | 6 (5–9.7) | 8 (5–12) | 0.07 |
BRPA borderline resectable pancreatic adenocarcinoma, LAPA locally advanced pancreatic adenocarcinoma.
Fig. 1. Study flowchart showing the treatments according to BRPA and LAPA populations.
BRPA borderline resectable pancreatic adenocarcinoma, LAPA locally advanced pancreatic adenocarcinoma, CRT chemoradiation therapy.
FOLFIRINOX induction treatment and safety
After a median of 7 cycles (5–11) of FOLFIRINOX, dose adaptation was required for 72.4% of patients, for 5FU (62.3%), oxaliplatin (54.7%) and/or irinotecan (48.0%). FOLFIRINOX was stopped because of toxicity in 12.1% of patients, including 2 toxic deaths (0.6%). Primary prophylaxis with G-CSF was used in 79.8% of cases (Supplementary Table 1). Main grade 3/4 toxicities were fatigue (15.0%), neutropenia (11.6%), nausea (8.6%) and diarrhoea (7.2%) (Table 2). Oxaliplatin-induced peripheral grade 2/3 neuropathy was observed in 35.4% of patients.
Table 2.
FOLFIRINOX-related toxicities according to CTCAE grading.
| None | G1 | G2 | G3 | G4 | Missing | |
|---|---|---|---|---|---|---|
| Nausea and vomiting | 98 (32.5%) | 103 (34.1%) | 75 (24.8%) | 25 (8.3%) | 1 (0.3%) | 28 (8.5%) |
| Diarrhoea | 106 (35.0%) | 101 (33.4%) | 74 (24.4%) | 21 (6.9%) | 1 (0.3%) | 27 (8.2%) |
| Hand–foot syndrome | 287 (96.0%) | 9 (3.0%) | 3 (1%) | — | — | 31 (9.4%) |
| Mucositis | 216 (75%) | 48 (16.7%) | 19 (6.6%) | 3 (1.0%) | 2 (0.7%) | 42 (12.7%) |
| Alopecia | 219 (78.5%) | 37 (13.2%) | 22 (7.9%) | 1 (0.4%) | — | 51 (15.4%) |
| Neutropenia | 204 (67.8%) | 28 (9.3%) | 34 (11.3%) | 25 (8.3%) | 10 (3.3%) | 29 (8.8%) |
| Thrombopenia | 220 (72.6%) | 49 (16.2%) | 26 (8.6%) | 7 (2.3%) | 1 (0.3%) | 27 (8.2%) |
| Anaemia | 181 (59.7%) | 95 (31.4%) | 20 (6.6%) | 7 (2.3%) | — | 27 (8.2%) |
| Neurotoxicity | 79 (26.2%) | 116 (38.4%) | 87 (28.8%) | 20 (6.6%) | — | 28 (8.5%) |
| Fatigue | 8 (5.5%) | 56 (38.4%) | 60 (41.1%) | 22 (15.0%) | — | 184 (55.7%) |
| Maximal toxicity | 16 (4.9%) | 53 (16.4%) | 151 (46.6%) | 89 (27.5%) | 15 (4.6%) | 6 (2%) |
Treatments
After FOLFIRINOX, at least one consolidation treatment was administered in 61.5% of patients, 58.4% in the LAPA group and 66.7% in the BRPA group (Table 3). A total of 153 patients (46.4%) received CRT after FOLFIRINOX, without any further treatment in this setting for 120 patients (36.4% of the overall population). Secondary resection was performed overall in 79 patients (23.9% of the overall population) and in 33/79 patients (41.8%) after CRT.
Table 3.
Response rate and treatments after induction FOLFIRINOX chemotherapy.
| N (%) | Whole population N = 330 | BRPA N = 102 | LAPA N = 226 | p | |
|---|---|---|---|---|---|
| Objective radiological response (RECIST 1.1) | Complete response | 11 (3.7) | 1 (1.1) | 10 (4.8) | 0.18 |
| Partial response | 77 (25.8) | 27 (30.3) | 50 (24.2) | ||
| Stable disease | 152 (51.0) | 48 (53.9) | 102 (49.3) | ||
| Progression | 58 (19.5) | 13 (14.6) | 45 (21.7) | ||
| Treatment after FOLFIRINOX | Yes | 201 (61.5) | 69 (66.7) | 129 (57.1) | 0.20 |
| Chemoradiotherapy alone | 120 (36.4) | 26 (25.5) | 94 (41.6) | <0.0001 | |
| Surgery | 79 (23.9) | 43 (42.1) | 35 (15.5) | ||
| With radiotherapy | 33 (41.8) | 17 (39.5) | 15 (42.9) | 0.77 | |
| Without radiotherapy | 46 (58.2) | 26 (60.5) | 20 (57.1) | ||
| Surgical exploration with no resection | 12 (3.6) | 6 (5.9) | 6 (2.6) | 0.14 | |
| Post-FOLFIRINOX radiotherapy | 153 (46.6) | 43 (42.6) | 109 (48.4) | 0.34 | |
| Dose (Gy) | Median (range) | 50 (49–54) | 50 (50–54) | 50 (48–54) | 0.85 |
| Post-FOLFIRINOX surgical resection | N = 79 | N = 43 | N = 35 | ||
| R0 resection | 59 (74.7) | 32 (74.4) | 25 (71.4) | 0.90 | |
| ypT0N0 | 7 (8.9) | 3 (7.0) | 4 (11.8) | 0.69 | |
| Post-operative complication | 30 (40.5) | 17 (39.5) | 12 (40) | 0.97 | |
| With radiotherapy | 12 (38.7) | 6 (35.3) | 5 (38.5) | ||
| Without radiotherapy | 18 (41.9) | 11 (42.3) | 7 (41.2) | ||
| Recurrence | After radiotherapy alone (n = 120) | 76 (63.3) | 12 (46.1) | 64 (68.1) | 0.03 |
| After surgery (n = 79) | 36 (45.6) | 22 (51.2) | 14 (40.0) | ||
| With radiotherapy | 10 (30.3) | 6 (35.3) | 4 (26.7) | ||
| Without radiotherapy | 26 (56.5) | 16 (61.5) | 10 (50.0) | ||
| Missing | 166 | 43 | 123 | ||
| Metastatic recurrence after consolidation treatment | 70 (61.9) | 22 (62.8) | 48 (61.5) | 0.51 |
BRPA borderline resectable pancreatic adenocarcinoma, LAPA locally advanced pancreatic adenocarcinoma.
Radiotherapy
One hundred and twenty patients received CRT alone after FOLFIRINOX, representing 25.5% of BRPA patients and 41.6% of LAPA patients (Table 3). The mean interval between the first FOLFIRINOX administration and the beginning of CRT was 5.5 months (SD: 4.1). The median dose of RT was 50 Gy (range 49–54) with no difference between the BRPA and LAPA groups. Concomitant chemotherapy was administered in 94.7% of patients, mainly with capecitabine 1650 mg/m2 per day.
Secondary surgery
Among the 79 patients who underwent surgery, n = 43 had BRPA (42.1% of the BRPA group) and n = 36 had LAPA (15.5% of the LAPA group) (Table 3). R0 resection was achieved in 59 patients (74.7%), and ypT0N0 tumours were found in 7 patients (8.9%).
In the BRPA group, R0 resection was obtained in 32 patients (74.4%) and ypT0N0 tumours were found in 3 (7.0%) patients. In the LAPA group, R0 resection was obtained in 25 patients (71.4%) and ypT0N0 tumours were found in 4 (11.8%) patients.
In the patients who underwent surgery, 40.5% had a surgical complication with no differences between the BRPA and LAPA groups (39.5 and 40.0%, respectively). The main complication was pancreatic fistula (20.0% of patients in both the groups). Pre-operative RT did not confer a higher risk of surgical complications (38.7% in the CRT group vs 41.9%, Table 3). There was no difference in terms of local involvement (arterial or venous) between patients with or without surgical complications.
Among the 79 patients who underwent surgery, 33 received pre-operative CRT (41.8%). In the LAPA group, 15 patients (42.9% of the 35 resected LAPA) had pre-operative CRT followed by surgery. In the BRPA group, 17 patients (39.5% of the 43 resected BRPA) had pre-operative CRT followed by surgery. Of note, 1 patient who received pre-operative CRT had an unclassified LAPA/BRPA status.
After surgery (n = 79), 57 patients (72.1%) received adjuvant chemotherapy and 4 (5.1%) adjuvant CRT (Supplementary Table 2). Adjuvant chemotherapy was mainly gemcitabine (59.6%) and FOLFIRINOX (24.6%). Patients with BRPA were more likely to receive adjuvant treatment (86.0 vs 58.8%, p = 0.007).
No treatment stoppage was planned in patients treated with FOLFIRINOX alone. In the FOLFIRINOX + CRT group, the time between last FOLFIRINOX injection and second-line treatment was 7.1 months (interquartile range (IQR): 4.8; 9.2) and it was 12.4 months (IQR: 9.1; 16.6) in the FOLFIRINOX + surgery group.
Survival and response endpoints
Objective response rate (ORR) was 29.5% and disease control rate (DCR) was 80.5% (Table 3). In the LAPA group, ORR and DCR were 29.0 and 78.3%, respectively. In the BRPA group, ORR and DCR were 31.4 and 85.3%, respectively. None of the study patient underwent CRT before 4.5 months of FOLFIRINOX and progression under FOLFIRINOX within this period was observed in 58 patients (11.8%). Meanwhile, among the 120 patients who underwent CRT without further surgery, progression within 3 months following CRT occurred in 30 patients (25.0%).
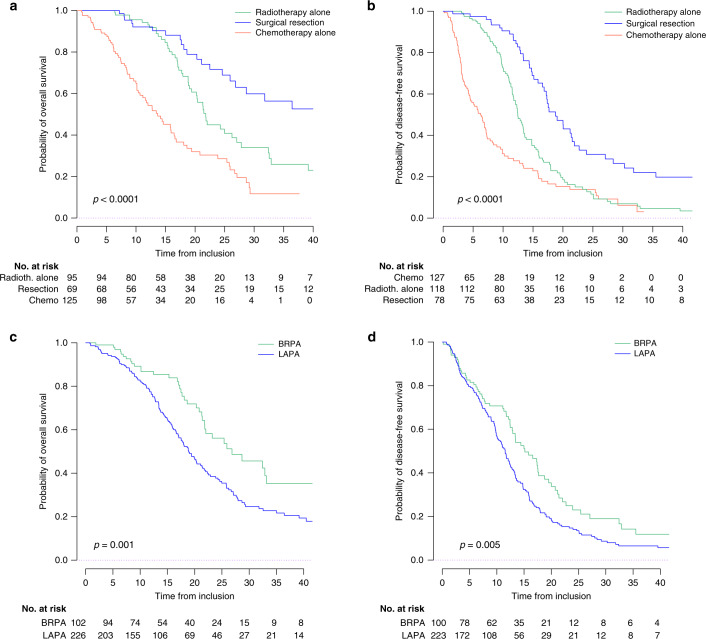
After a median follow-up of 26.7 months, median OS was 21.4 months (95% CI: 19.1–24.3) for the overall population with 1-year, 2-year and 3-year OS rates of 80.1% (95% CI: 75.6–84.8), 43.2% (95% CI: 36.5–49.7), and 25.2% (95% CI: 19.4–32.7). Local involvement (BRPA or LAPA) was not known for 2 patients who were excluded from the survival analysis. Median PFS was 12.4 months (95% CI: 11.5–13.4) in the overall population with 1-year, 2-year and 3-year PFS rates of 52.5% (95% CI: 47.1–58.6), 17.1% (95% CI: 12.9–22.7) and 8.0% (95% CI: 5.0–12.8). Seven patients were not evaluable for PFS. In the LAPA and BRPA groups, median OS were 18.9 months (95% CI: 17.1–21.8) and 26.8 months (95% CI: 21.8–NR) (p = 0.001), and median PFS were 11.5 months (95% CI: 10.1–12.7) and 15.1 months (95% CI: 12.9–18.8) (p = 0.005), respectively (Supplementary Table 3, Fig. 2 and Supplementary Figs. 1, 2 and 3).
Fig. 2. Survival curves in the overall study population.
Kaplan–Meier curves displaying survival according to the treatment received (a OS, b PFS) and according to the BRPA/LAPA status (c OS, d PFS). BRPA borderline resectable pancreatic adenocarcinoma, LAPA locally advanced pancreatic adenocarcinoma. Number of patients at risk varies due to missing data.
For patients who underwent secondary resection, DFS was 18.8 months (95% CI: 17.2–22.7) in the overall population and 20.0 months (95% CI: 14.8–30.3) and 17.7 months (95% CI: 17.3–27.1) in the LAPA and BRPA groups, respectively.
Patients receiving CRT before surgery had better DFS (p < 0.0001), but no difference was seen for OS (p = 0.20) (Supplementary Fig. 3). In BRPA patients, a better DFS (23.9 vs 16.6 months, p = 0.01) and a trend to a better OS (NR vs 28.7 months, p = 0.09) were seen after pre-operative CRT (Supplementary Table 3). These differences were not seen in LAPA patients (DFS: 19.0 vs 22.1 months, p = 0.20; OS: NR vs 31.8 months, p = 0.70).
Factors associated with survival
In the univariate analysis, local involvement (LAPA vs BRPA), biliary stent, PS (0 vs 1 vs 2), and type of treatment (FOLFIRINOX alone vs FOLFIRINOX followed by CRT vs FOLFIRINOX followed by surgery) were significantly associated with OS and PFS in the LAPA group. In the BRPA group, the univariate analysis showed that tumour location (body vs isthmus vs tail vs head), PS and type of treatment were associated with OS and tumour location, type of treatment and pre-treatment CA 19-9 with PFS (Supplementary Tables 4 and 5).
In the LAPA group, the multivariate Cox model analysis showed that consolidation CRT alone (hazard ratio (HR) for OS: 0.41, 95% CI: 0.28–0.61; and HR for PFS: 0.47, 95% CI: 0.33–0.67) and secondary resection preceded or not by CRT (HR for OS: 0.21, 95% CI: 0.11–0.41; and HR for PFS: 0.21, 95% CI: 0.12–0.36) were associated with better OS and PFS (p < 0.0001; Table 4). Venous involvement alone was associated with worse OS and PFS in the LAPA group. There was a better OS for patients with a good PS at FOLFIRINOX initiation.
Table 4.
Multivariate analysis (Cox regression model) of factors associated with OS and PFS.
| BRPA | LAPA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall survival (n = 101) | Progression-free survival (n = 99) | Overall survival (n = 210) | Progression-free survival (n = 210) | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Local involvement | ||||||||||||
| Arterial alone | 1 | — | 0.0002 | 1 | — | 0.03 | ||||||
| Venous alone | 2.33 | 1.53–3.56 | 1.52 | 1.05–2.21 | ||||||||
| Arterial and venous | 1.10 | 0.68–1.76 | 0.90 | 0.60–1.37 | ||||||||
| Biliary stent (yes vs no) | 1.36 | 0.93–2.00 | 0.11 | 1.19 | 0.86–1.65 | 0.29 | ||||||
| Tumour location | ||||||||||||
| Body | 1 | 0.02 | 1 | 0.11 | ||||||||
| Head | 0.33 | 0.15–0.70 | 0.57 | 0.31–1.08 | ||||||||
| Tail | 0.39 | 0.10–1.51 | 0.33 | 0.09–1.18 | ||||||||
| Performance status | ||||||||||||
| 0 | 1 | 1 | 1 | 1 | ||||||||
| 1 | 0.82 | 0.40–1.68 | 0.61 | 1.13 | 0.65–1.96 | 0.78 | 1.23 | 0.84–1.81 | 0.04 | 1.11 | 0.79–1.55 | 0.23 |
| 2 | 1.53 | 0.42–5.58 | 1.52 | 0.44–5.30 | 2.87 | 1.25–6.57 | 1.88 | 0.91–3.90 | ||||
| Type of treatment | ||||||||||||
| Chemo alone | 1 | 1 | 1 | 1 | ||||||||
| Chemo and CRT | 0.59 | 0.26–1.37 | 0.01 | 0.63 | 0.33–1.17 | 0.001 | 0.41 | 0.28–0.61 | <0.0001 | 0.47 | 0.33–0.67 | <0.0001 |
| Chemo +/− RT and secondary resection | 0.26 | 0.11–0.63 | 0.32 | 0.18–0.60 | 0.21 | 0.11–0.41 | 0.21 | 0.12–0.36 | ||||
BRPA borderline resectable pancreatic adenocarcinoma, LAPA locally advanced pancreatic adenocarcinoma, HR hazard ratio, 95% CI 95% confidence interval, CRT chemoradiation therapy.
In the BRPA group, the multivariate Cox model analysis showed that consolidation CRT alone (HR for OS: 0.59, 95% CI: 0.26–1.37; and HR for PFS: 0.63, 95% CI: 0.33–1.17) and secondary resection preceded or not by CRT (HR for OS: 0.26, 95% CI: 0.11–0.63; and HR for PFS: 0.32, 95% CI: 0.18–0.60) were associated with better OS and PFS (p = 0.01 for OS and p = 0.001 for PFS) (Table 4).
Discussion
In this study, we provide real-world data on patients receiving induction FOLFIRINOX for locally advanced or borderline resectable PA. This is to our knowledge one of the largest series reported to date. We found that FOLFIRINOX induction therapy followed by CRT and/or surgery in some patients is associated with a DCR of 80.0%, a median OS of 21.4 months and a median PFS of 12.4 months. Finally, resection rates were 42.1% in the BRPA population and 15.5% in the LAPA population, with median OS and DFS of 36.4 and 18.8 months, respectively, in secondary resected patients.
This study is an update of a previously published study in 77 patients.14 As compared to our previous publication, we found here a slightly lower resection rate than before and those reported in Phase 2 trials.16,17 This lower rate may be explained by the larger number of participating centres and the inclusion of all consecutive patients, not highly selected as in prospective trials. Moreover, the resection rate of 23.9% is in accordance with the meta-analysis published by Suker et al. that found a 25.9% resection rate after FOLFIRINOX induction treatment.8 More recently, Maggino et al. also showed in a population of 260 patients very similar results with resection rates of 12.4% in LAPA and 33.6% in BRPA patients. In this study, FOLFIRINOX was compared to gemcitabine alone, GEMOX or gemcitabine–nabpaclitaxel.18 FOLFIRINOX seemed to have the best resection rates, encouraging the use of this triplet regimen in this setting. Other less well-defined series showed similar results.15,19–21 ORR and DCR in our study were 29 and 80%, respectively, which is in accordance with previously published studies,14 and close to what is observed when using FOLFIRINOX in the metastatic setting.12
In the present work, complementary CRT and surgery were independently associated with better survivals in multivariable analyses. Though no benefit for pre-operative CRT was observed in the overall population who underwent secondary resection, patients with BRPA seemed to exhibit better OS and DFS when treated with CRT pre-operatively. Our study’s lack of power may have resulted in this trend to significance. In the recently published PREOPANC Phase 3 trial, pre-operative gemcitabine and CRT were associated with an increase of OS and DFS in comparison to front-line surgery in the subgroup of patients with BRPA, whereas no benefit was observed in the subgroup of patients with resectable disease.11 In this study, median OS and DFS for BRPA after CRT plus surgery were 17.6 and 6.3 months, respectively. Though cross-study comparison remains debatable and our study was not a prospective randomised trial, the results reported here seem to compare favourably with those of the PREOPANC study. FOLFIRINOX followed by CRT in patients with controlled disease seems a promising option for BRPA and should now be evaluated in prospective randomised trials, as the ongoing PANDAS trial (NCT02676349), testing FOLFIRINOX 6 courses + /− CRT in patients with BRPA, or the PREOPANC 2 trial (NTR7292).
In the LAP 07 trial, which provided randomised data concerning consolidation CRT after gemcitabine + /− erlotinib in 446 patients with LAPA,22 no benefit was observed in terms of OS in the CRT group and only 12 patients underwent secondary resection. In our study, 15.5% of patients with LAPA underwent surgical resection and 41.6% had consolidation CRT. In addition, an 8-month longer median OS was observed in patients receiving consolidation CRT (HR: 0.41; 95% CI: 0.28–0.61). These differences may be due to a more effective induction treatment with FOLFIRINOX as already reported by others.18 With all the limitations of non-randomised studies, here again a signal of better outcomes when using FOLFIRINOX as an induction treatment before CRT should prompt enrolment of patients in clinical trials to test these questions, as in the ongoing NEOPAN trial testing gemcitabine vs FOLFIRINOX + /− CRT in LAPA patients.
In this study, we used standard normofractionated schedule (1.8–2 Gy per fraction). The use of SBRT with dose-escalated RT treatment could also be an interesting approach for selected patients: it could improve local control with the delivery of higher biologically effective doses to the tumour.23 Several studies suggested interesting results in terms of feasibility, safety and efficacy, providing high local control (around 85% at 2 years on average) and improved R0 resection rates but little or no impact on survival.24–27 Up to this day, there is no randomised study comparing these radiation therapy schemes.
Considering tolerability, we found that FOLFIRINOX was associated with G3/4 toxicity in 33.5% of cases. As compared to Suker et al., who reported grade 3/4 toxicity in 69.4% of cases in their meta-analysis, our better numbers may be not only due to a lower quality of data collection due to the nature of this cohort but also to a FOLFIRINOX regimen without 5FU bolus injection in the vast majority of patients, a higher use of G-CSF as primary prophylaxis and training in most centres for the past 10 years in the use of FOLFIRINOX in PAC patients. Adverse events were easily managed in daily practice in most patients, and only 12.7% of the patients had to stop treatment because of toxicity.
Though a large number of patients were enrolled in this retrospective cohort, our study has some limitations. First, non-resectability was defined by each centre. Nevertheless, in France MDT meetings are mandatory and each patient was assessed by a GI oncologist, a radiologist and a pancreatic surgeon. No centralised radiological review was conducted to confirm non-resectability and the stage at diagnosis, resulting in possible misclassification of some LAPA or BRPA patients. However, this reflects the variability of decisions among different centres and real-world patient care and all centres participating in the study are secondary or tertiary centres specialised in pancreatic surgery. Second, consolidation treatments (RT, surgery) were decided according to the characteristics of each patient and during MDT meetings in each centre with no randomisation. Finally, retrospective collection of data leads to missing information, but the rate of missing data was low for most variables.
Conclusion
This retrospective large-scale study shows that induction FOLFIRINOX + /− RT has an acceptable safety profile and seems to be an effective option for LAPA and BRPA, with an ORR of 29% and secondary resection in 23.9% of cases. The median OS and PFS of 21.4 and 12.4 months, respectively, are also longer than those reported with other chemotherapeutic regimens in this setting, especially gemcitabine alone. There was a signal towards improved outcomes for the addition of consolidation CRT before surgery and after induction FOLFIRINOX, especially for BRPA patients. CRT remains to be validated in prospective trials both in unresectable patients and in the pre-operative setting.
Supplementary information
Acknowledgements
The authors would like to acknowledge David Marsh (language review).
Author contributions
E.A.: study design, statistical analysis, manuscript writing and editing. L. Marthey: study design, data collection, manuscript writing, R.A., L. Mas, E. Francois, A.S., A.S.C., A.V., T.L., V.H., C.d.L.F., M.S., F.K., J.F., R.C., E. Fabiano, F.L., N.W.: data collection, manuscript writing, J.B.B., D.T.: study design, manuscript writing, J.T.: study design, data analysis, manuscript writing.
Ethics approval and consent to participate
No informed consent was needed for this observational study, as stated by the French ethics committee consulted prior to the beginning of the work.
Data availability
Data are available from J.T. at reasonable request.
Competing interests
E.A.: Travel expenses: Mundipharma. Lectures and educational activities: Sanofi Genzymes, Lilly-Oncology. J.T.: Consulting or advisory role: Roche, Merck KGaA, Darmstadt, Germany, Amgen, Celgene, Eli Lilly, Servier, Sirtex Medical, Merck Sharp & Dohme, Pierre Fabre. Speakers’ Bureau: Servier, Amgen, Roche/Genentech, Sanofi, Merck KGaA, Darmstadt, Germany, Eli Lilly, Merck Sharp & Dohme, Pierre Fabre. The other authors have nothing to declare.
Funding information
This study was not funded.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01341-w.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Institut National du Cancer. Les cancers en France en 2015, l’essentiel des faits et chiffres. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers-en-France-en-2015-L-essentiel-des-faits-et-chiffres (2016).
- 3.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin. Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 5.Katz MHG, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann. Surg. Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl Compr. Cancer Netw. 2019;17:202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 8.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 10.Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44:515–521. doi: 10.1097/MPA.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 11.Versteijne, E., Suker, M., Groothuis, K., Akkermans-Vogelaar, J. M., Besselink, M. G., Bonsing, B. A. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38, 1763–1773 (2020). [DOI] [PMC free article] [PubMed]
- 12.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul J-L, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 14.Marthey L, Sa-Cunha A, Blanc JF, Gauthier M, Cueff A, Francois E, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann. Surg. Oncol. 2015;22:295–301. doi: 10.1245/s10434-014-3898-9. [DOI] [PubMed] [Google Scholar]
- 15.Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199. doi: 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz MHG, Shi Q, Ahmad SA, Herman JM, Marsh R, de W, Collisson E, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151:e161137. doi: 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggino, L., Malleo, G., Marchegiani, G., Viviani, E., Nessi, C., Ciprani, D. et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 154, 932–942 (2019). [DOI] [PMC free article] [PubMed]
- 19.Conroy T, Paillot B, François E, Bugat R, Jacob J-H, Stein U, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer-a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J. Clin. Oncol. 2005;23:1228–1236. doi: 10.1200/JCO.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–548. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunturu KS, Yao X, Cong X, Thumar JR, Hochster HS, Stein SM, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med. Oncol. 2013;30:361. doi: 10.1007/s12032-012-0361-2. [DOI] [PubMed] [Google Scholar]
- 22.Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 23.Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin. Radiat. Oncol. 2014;24:140–147. doi: 10.1016/j.semradonc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Palta M, Czito BG, Duffy E, Malicki M, Niedzwiecki D, Abbruzzese J, et al. A phase II trial of neoadjuvant gemcitabine/nab-paclitaxel and SBRT for potentially resectable pancreas cancer: an evaluation of acute toxicity. J. Clin. Oncol. 2018;36:4121. doi: 10.1200/JCO.2018.36.15_suppl.4121. [DOI] [Google Scholar]
- 27.Quan K, Sutera P, Xu K, Bernard ME, Burton SA, Wegner RE, et al. Results of a prospective phase 2 clinical trial of induction gemcitabine/capecitabine followed by stereotactic ablative radiation therapy in borderline resectable or locally advanced pancreatic adenocarcinoma. Pract. Radiat. Oncol. 2018;8:95–106. doi: 10.1016/j.prro.2017.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from J.T. at reasonable request.