Fig. 1. The molecular composition of mitochondria-ER contact sites: several sets of complexes tether the mitochondria and ER.
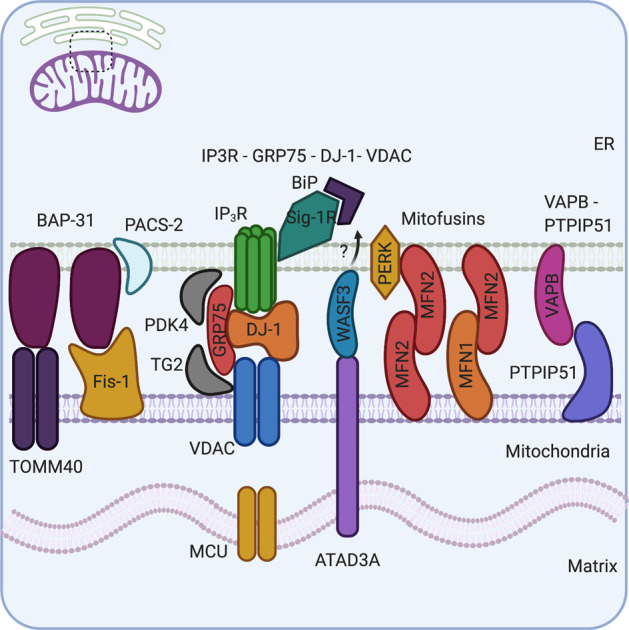
BAP-31 in the ER interacts with Fis-1 and TOMM40 in the OMM. PACS-2, a multifunctional sorting protein in the ER, regulates BAP-31 MERCS interactions. IP3R in the ER and VDAC in the OMM form a tetramer complex with regulatory proteins, GRP75 and DJ-1, to control calcium (Ca2+) transfer into the mitochondria. Further regulatory proteins such as TG2 and PDK-4 also bind GRP75–IP3R–VDAC complex regulating MERCS. Sig-1R accumulates in MERCS and can stabilise IP3R in MERCS, but also interacts with chaperone protein BIP in the ER lumen. ATAD3A can cross the IMM and OMM to interact with BiP in the ER via the cytosolic protein WASF3 and other unknown proteins. MFN2 is located in both the ER and mitochondrial membrane and can homodimerise with itself or heterodimerise with MFN1 in the OMM. MFN2 has known interactions with PERK, which are required for progression through the UPR. Finally, VAPB in the ER membrane and PTPIP51 in the OMM interact and directly regulate MERCS size and length and act as a physical tether.
