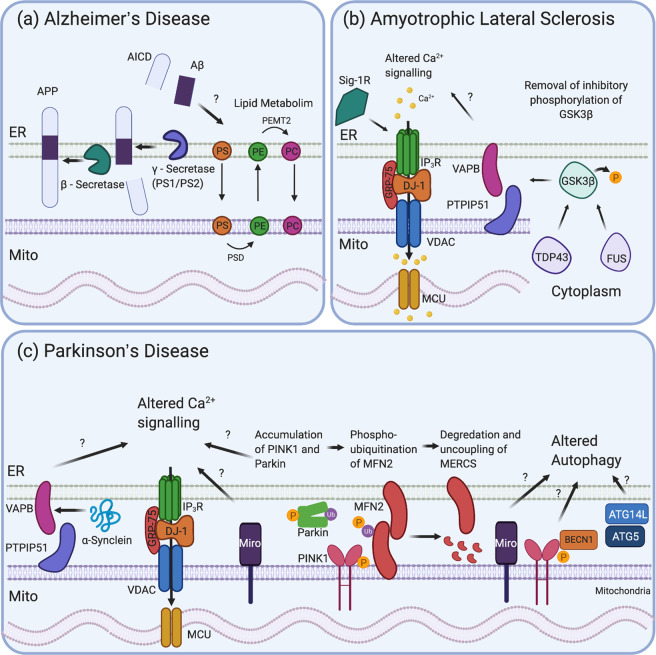
Fig. 3. Mitochondrial ER contact sites in neurodegeneration.
a AD: amyloid precursor protein (APP), along with its metabolites, and β- and γ-secretase enzymes are found in MERCS. The APP is first cleaved by β-secretase and then γ-secretase to release Aβ. Aβ has been shown to alter lipid metabolism at MERCS. b ALS: TDP-43 and FUS in the cytoplasm activate GSK3β by dephosphorylating it at cysteine 9. GSK3β can then disrupt VAPB and PTPIP51 binding, uncoupling the ER from the mitochondria and altering Ca2+ signalling. Sig-1R can bind IP3R, stabilising it in the membrane, while loss of Sig-1R can result in ALS-like symptoms in mice and uncoupling of MERCS. c PD: WT or mutated α-Synuclein interacts with VAPB altering its binding to PTPIP51, disrupting MERCS and Ca2+ signalling. Miro is present in MERCS and can disrupt Ca2+ signalling and autophagy. PINK1 and Parkin, under mitophagy induction, have been shown to localise to MERCS and also impact on Ca2+ signalling and to promote the phosphoubiquitination of MFN2, resulting in its degradation and the uncoupling of the mitochondria from the ER. PINK1 and Miro have also been associated with altered mitophagy as PINK1 interacts with BECN1. Key autophagy genes (e.g., ATG14L and ATG5) are also present in MERCS and impact on mitophagy, a pathway dysregulated in PD.