Figure 2.
Spike ΔH69/V70 does not reduce sensitivity to neutralizing antibodies
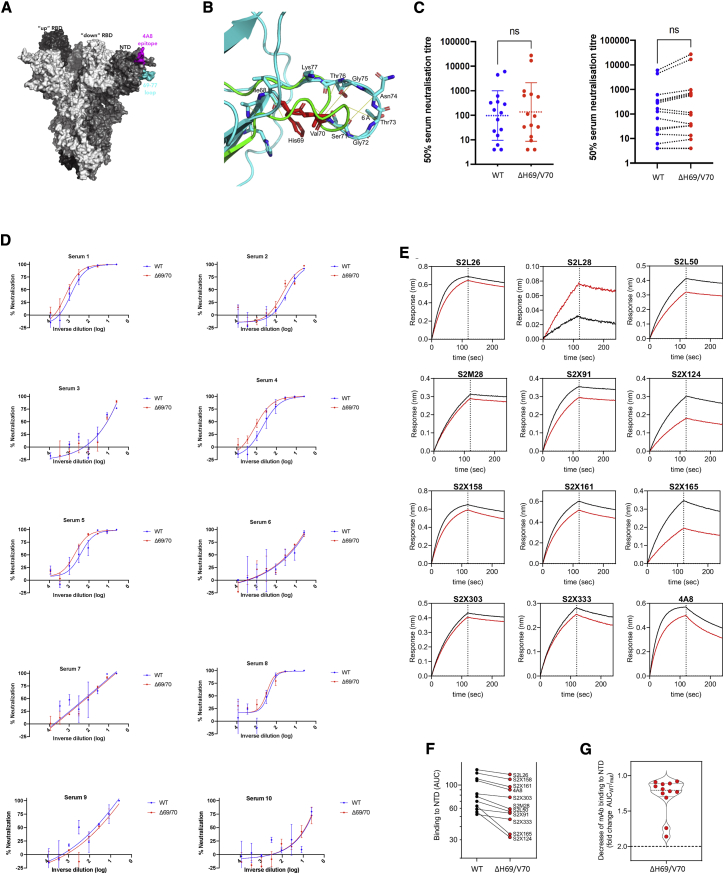
(A) Surface representation of the spike homotrimer in the open conformation (PDB: 7C2L), with each monomer shown in different shades of gray. On the monomer shown positioned to the right, the exposed loop consisting of residues 69–77 is shown in cyan and the neutralizing antibody (4A8)-binding NTD epitope in magenta.
(B) Prediction of conformational change in the spike NTD because of deletion of residues His69 and Val70. The pre-deletion structure is shown in cyan, except for residues 69 and 70, which are shown in red. The predicted post-deletion structure is shown in green. Residues 66–77 of the pre-deletion structure are shown in stick representation and colored by atom (nitrogen in blue, oxygen in coral). Yellow lines connect aligned residues 66–77 of the pre- and post-deletion structures, and the distance of 6 Å between aligned alpha carbons of Thr73 in the pre- and post-deletion conformation is labeled.
(C) Neutralization of spike ΔH69/V70 PV and WT (D614G background) by convalescent sera from 15 donors. GMT (geometric mean titer) with SD presented is representative of two independent experiments, each with two technical repeats. Wilcoxon matched-pairs signed-rank test; ns, not significant.
(D) Ten example neutralization curves. Indicated is serum log10 inverse dilution against percent neutralization. Data points represent means of technical replicates, and error bars represent SD. Curves are representative of two independent experiments.
(E–G) Kinetics of binding to WT and ΔH69/V70 NTD of 12 NTD-specific mAbs.
(E) Biolayer interferometry analysis of binding to wild-type (WT; black) and WT ΔH69/V70 (red) NTDs by 12 NTD-targeting mAbs. Dotted lines separate the association phase from the dissociation phase. Shown is 1 of 2 independent experiments.
(F) Side-by-side comparison of binding to WT (black) and ΔH69/V70 (red) NTDs by 11 NTD-targeting mAbs. Binding is shown as area under the curve (AUC). The S2L28 mAb is not shown because of too little response measured (<0.10 nm).
(G) Binding to NTD of the 11 mAbs shown in (B), expressed as fold change of the AUC of the WT compared with ΔH69/V70.
Data are representative of two independent experiments.