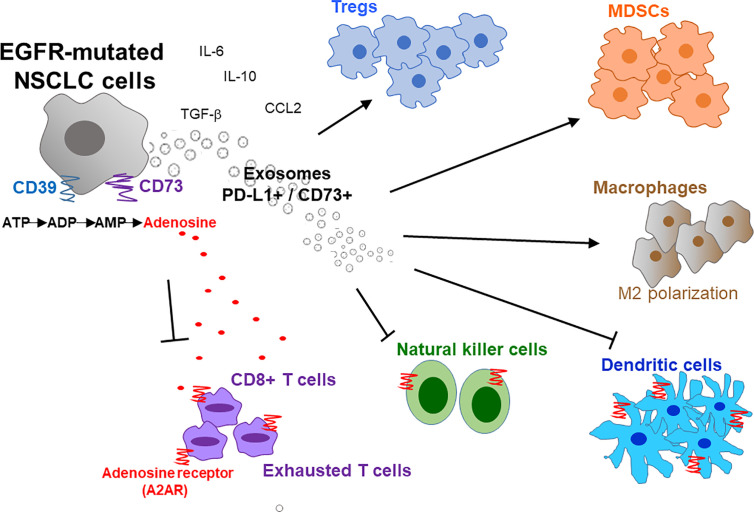
Figure 1.
Immunosuppressive tumor microenvironment (TME) in EGFR-mutated NSCLC. EGFR mutations promote an immunosuppressive TME by interfering with several intracellular pathways and modulating immune accessory cells including tumor-infiltrating lymphocytes (TILs), natural killer cells (NK), T-regulatory cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs). Overexpression of CD39/CD73 in EGFR-mutated NSCLC induces high extracellular production and release of adenosine that inhibit the activity of innate and adaptive immune system cells and endothelial cells in TME. Activation of CD39 triggers the de-phosphorylation of ATP to ADP, and subsequently to AMP. On the other hand, CD73 catalyzes the hydrolysis of AMP to adenosine and phosphate. The increased level of extracellular adenosine bind to A2A adenosine receptor (A2AR) expressed by both adaptive and innate immunity, thereby inhibiting the activity of various immune cells. Moreover, exosomes secreted from EGFR-mutated NSCLC cells also increase PD-L1+/CD73+ expression and extracellular adenosine release to promote immunosuppression. IL, interleukin; M2, macrophages 2; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; CCL2, C-C motif chemokine ligand 2.