Abstract
Objectives
There is considerable overlap in risk profiles between chronic low back pain with radiculopathy (CLBPR) and cardiovascular health among older adults; obesity and smoking are related to both conditions and may largely drive the potential relationship. We sought to explore the impact of CLBPR on cardiovascular health outcomes, independent of body mass index (BMI) and current smoking status.
Methods
Age- and sex-matched older adults (60–85 years of age) with (n = 21) and without (n = 21) CLBPR were recruited. Current smokers were excluded. Blood samples were collected to measure cholesterol levels and pro-inflammatory markers (i.e., C-reactive protein and interleukin-6). Vascular endothelial function, a marker of cardiovascular health, was evaluated by measuring brachial artery flow-mediated dilation (FMD). General linear models with multifactorial designs were evaluated; group membership, BMI, education, and their respective two-way interaction terms were included as independent variables.
Results
Older adults with CLBPR had significantly higher BMIs (P = 0.004) and lower educational levels (P = 0.013) than did those without pain. There was a significant group-by-education interaction effect (P = 0.049) for endothelial function. Older adults without pain who were highly educated had higher FMD values, indicating better endothelial function (9.2%), whereas the following combinations all had lower FMD values: no pain plus low education, CLBPR plus high education, and CLBPR plus low education (5.9%, 6.1%, and 6.6%, respectively).
Conclusions
Among older adults, CLBPR is linked with worse endothelial function, regardless of educational level and independent of BMI and smoking. These findings suggest that older adults with CLBPR may be at a higher risk of cardiovascular disease.
Keywords: Cardiovascular, Low Back Pain, Radiculopathy, Sciatica, Older Adults
Introduction
Chronic low back pain with radiculopathy (CLBPR) (i.e., pain that radiates from the lumbar spine into the leg[s]) is common among older adults [1, 2], as age augments the degenerative processes that underlie this condition. Older adults often undergo senescent changes in several body systems that not only increase the risk of persistent pain, but also increase the risk of poor health outcomes [3]. Experts have recently called for research to enhance our understanding of the consequences of low back pain–related conditions among older adults [4], as CLBPR leads to greater deficits in physical function and higher levels of disability than those seen in individuals who are either free of pain or have localized pain [2]. Nevertheless, our understanding of how CLBPR affects other important downstream health outcomes, such as cardiovascular disease, is limited.
Cardiovascular disease is an important health outcome to consider in the context of CLBPR, as it is the leading cause of death in the world [5]. Furthermore, there appears to be significant overlap between the two conditions. Prior work has shown that the underlying physiological mechanisms for the development and progression of CLBPR and cardiovascular disease may emerge from similar pathways. Radiculopathy is associated with chronic inflammation through pro-inflammatory markers, including C-reactive protein (CRP) and interleukin-6 (IL-6) [6]; inflammation plays a significant role in the development and progression of atherosclerosis [7–9]. Interestingly, other work has also indicated that cardiovascular changes may be responsible, in part, for causing CLBPR: Korkiakoski et al. [10] showed that diminished blood flow due to atherosclerosis in the lumbar arteries was associated with a history of CLBPR. Indeed, it is unsurprising that people with chronic pain-related conditions, like CLBPR, are at a higher risk of cardiovascular disease–related deaths [11].
However, the relationship between CLBPR and cardiovascular health is likely complex. Individuals with CLBPR are more likely to be obese and use tobacco [12–15]. Obesity and tobacco use are two of the strongest risk factors for cardiovascular disease [16] and, therefore, may largely explain any link between CLBPR and cardiovascular health. Thus, it is unclear whether older adults with CLBPR have worse cardiovascular health than their pain-free counterparts, independent of these traditional risk factors. If a relationship between CLBPR and cardiovascular heath persists despite obesity and tobacco use, then screening for these two well-known risk factors may not be sufficient for older adults with CLBPR.
In this study, our primary objective was to determine whether older adults with CLBPR had worse cardiovascular health than those without CLBPR, independent of obesity and tobacco use. We considered traditional clinical measures of cardiovascular health, including blood pressure and blood lipid panels (i.e., cholesterol and triglyceride measures). We also considered less traditional, yet important, markers of cardiovascular health, including pro-inflammatory markers (i.e., CRP and IL-6) and vascular endothelial function. We hypothesized that older adults with CLBPR would exhibit poorer cardiovascular health on the aforementioned measures than would pain-free older adults.
Methods
Participants and Study Overview
Forty-two community-dwelling, cognitively intact (i.e., Folstein Mini-Mental State Exam score ≥18) [17] older adults, 60–85 years of age, with (n = 21) and without CLBPR (n = 21) were recruited. Participants were recruited from local newspaper advertisements, health fairs, and senior centers from October 2015 to June 2016. All on-site procedures were performed after individuals signed an informed consent approved by the University of Delaware Institutional Review Board.
Individuals were included in the CLBPR group if they had low back pain of at least “moderate intensity” (>3/10) that occurred ≥4 days/week and was ≥3 months in duration, which is consistent with prior studies that have recruited older adults with chronic low back pain [18, 19]. Also, for inclusion, participants must have reported that they had radicular symptoms (i.e., pain/symptoms in their leg[s] related to their back that consistently worsened with upright posture and walking and improved with sitting [20]). Individuals were excluded if they had “red flag” signs or symptoms indicative of a serious underlying condition (e.g., bowel or bladder changes, abnormal sensation in the groin or buttock region, fever associated with their low back pain), had experienced a recent physically traumatic event (i.e., fall, surgery, motor vehicle accident), had severely limited mobility (i.e., needed more than a single-point cane for performance testing), had been diagnosed with a progressive neurological condition, or had been diagnosed with a systemic inflammatory disease (e.g., rheumatoid arthritis). Furthermore, to ensure that differences in comparisons between groups were not largely driven by tobacco use or body mass, all individuals were excluded if they were current cigarette smokers or had a body mass index (BMI) ≥35 kg/m2.
Pain-free older adults were included if they matched a participant in the CLBPR group on age (±5 years) and sex and they reported no low back pain within the previous year and no significant (i.e., >2/10 pain intensity) areas of pain at the time of screening. Pain-free older adults were excluded according to the same criteria as those with CLBPR.
To control for the direct and short-term impact that diet, exercise, and medications can have on physiological and biological measures, several precautions were taken. Before the study visit, participants fasted for 12 hours, and they abstained from exercise, caffeine consumption, and alcohol consumption for 24 hours. Furthermore, on the day of the testing, participants withheld their morning medications until testing was completed.
Descriptive Characteristics
Participants reported their age, sex, and educational level (i.e., graduated college; yes/no). Although current smokers were excluded, former smokers reported their smoking history in pack-years. Anthropometric measurements (i.e., height, weight, waist circumference) were taken, and BMI was calculated [21]. The modified Oswestry Disability Questionnaire was used to characterize low back pain–related disability among older adults with CLBPR; higher scores indicate greater disability [22]. Older adults with CLBPR also reported the duration of their symptoms.
Cardiovascular Health Measures
Brachial systolic and diastolic blood pressures were measured by an automated oscillometric sphygmomanometer (GE Medical Systems Dinamap Dash 2000, Milwaukee, Wisconsin) in the clinical laboratory, before the evaluation session. Blood pressure readings were taken on both arms and averaged to arrive at a single value for each systolic and diastolic measurement.
A venous blood sample was collected for measurement of a lipid panel and pro-inflammatory biomarkers. Lipid panels were sent to a clinical laboratory for analysis of triglycerides, as well as total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol levels. Measurements of pro-inflammatory biomarkers, CRP and high-sensitivity IL-6, were performed on serum samples that had been stored at −80 C. The biochemical analyses were performed via solid-phase sandwich Quantikine ELISA kits according to the manufacturer’s protocols (R&D Systems, Minneapolis, Minnesota); all samples were assayed in duplicate. Serum inflammatory markers were reported as the average of the two samples in milligrams per liter (mg/L) and picograms per milliliter (pg/mL) for CRP and IL-6, respectively. Higher concentrations indicated greater inflammation (i.e., worse cardiovascular health).
We assessed vascular health with brachial artery flow-mediated dilation (FMD), a measure of conduit artery function. Brachial artery FMD, the most commonly used noninvasive measure of endothelial function, is highly correlated with coronary circulation [23, 24], has a significant predictive value for risk of future cardiovascular events in older adults [25], and is considered an independent cardiovascular disease risk factor from other conditions, such as hypertension [26, 27].
Brachial artery FMD was performed according to established guidelines in order to assess endothelium-dependent dilation [28, 29]. Participants were supine, with their dominant arm supported at heart level. Longitudinal images of the brachial artery were obtained proximal to the antecubital fossa with a high-frequency (60-Hz) ultrasound transducer. Continuous, two-dimensional images were obtained at baseline with B-mode Doppler ultrasound (TerasonUSmart 3200 T, Teratech Corporation, Burlington, Massachusetts) to assess arterial diameter and flow velocity. Reactive hyperemia was then induced by the rapid inflation of a pneumatic cuff around the forearm to 220 mm Hg for 5 minutes; continuous arterial diameter and flow velocity measurements were made for 2 minutes after cuff deflation. All measurements were stored digitally and analyzed offline. Results of FMD were expressed as percentage change in arterial diameter from baseline diameter, where higher values indicated better vascular endothelial function. Percent change in FMD was calculated by the following formula:
Statistical Analyses
All statistical analyses were performed in IBM SPSS Version 26 (IBM Corporation, Armonk, New York). To determine between-group differences for descriptive characteristics and cardiovascular health measures, t tests and chi-squared tests were used for continuous and categorical variables, respectively. If continuous data violated parametric assumptions (i.e., non-normal distribution or lack of homogeneity of variance), Mann-Whitney U tests were used for between-group comparisons.
General linear models were used to determine the relationship between group membership and cardiovascular health outcomes, while accounting for other important factors. Older adults with CLBPR had significantly lower levels of education (P = 0.013) and greater BMIs (P = 0.010) than those of individuals who had no pain, despite the exclusion of individuals with a BMI ≥35 kg/m2. In addition to BMI, lower educational level is strongly associated with chronic pain among older adults [30]; as such, they were entered into the model as covariates. Models including the three main effects of group, BMI, and education and the three two-way interactions were investigated: group by education, group by BMI, and education by BMI. For the purposes of this study, however, we were interested primarily in the group main and interaction effects. If models violated parametric assumptions, we removed any outliers; if no outliers were present, we transformed the data by using an approach that fit the direction and degree of the skew observed. These two strategies satisfied all parametric test assumptions, and thus, nonparametric tests were not needed. Partial eta-squared values (i.e., the proportion of the variance in the dependent variable explained by a single factor) and the associated P values were examined. For all analyses, α = 0.050.
Results
Unadjusted between-group comparisons of descriptive and cardiovascular health characteristics are shown in Table 1. Compared with older adults without pain, participants with CLBPR were less likely to have graduated from college (P = 0.013) and exhibited higher BMIs (P = 0.004), higher concentrations of IL-6 (P = 0.016), and lower FMD values (P = 0.009). Older adults with CLBPR also had a symptom history of approximately 4 years, with Oswestry scores that corresponded to a moderate degree of low back pain–related disability [31]. Blood pressure values were similar between groups. Also, there were no differences in triglyceride or cholesterol values between groups, and these values were considered to be in a normal, healthy range.
Table 1.
Descriptive characteristics by group
| CLBPR (n = 21) | No Pain (n = 21) | P Value | |
|---|---|---|---|
| Age, y | 71.8 ± 4.4 | 71.0 ± 5.6 | 0.627 |
| Sex (female) | 13 (61.9) | 13 (61.9) | 1.000 |
| Education (graduated college) | 8 (38.1) | 16 (76.2) | 0.013 |
| Smoking history, pack-years* | 0.5 ± 28.5 | 0.0 ± 10.0 | 0.357 |
| BMI, kg/m2* | 26.7 ± 2.8 | 24.0 ± 5.0 | 0.004 |
| Waist circumference, cm | 86.8 ± 49.4 | 69.8 ± 59.6 | 0.330 |
| CLBPR symptom duration, y | 4.1 ± 5.5 | – | – |
| Oswestry (0–100%) | 36.3 ± 15.9 | – | – |
| Average SBP, mm Hg | 141.0 ± 18.8 | 136.6 ± 16.5 | 0.429 |
| Average DBP, mm Hg | 72.3 ± 9.1 | 70.6 ± 9.2 | 0.558 |
| Total cholesterol, mg/dL | 185.7 ± 34.7 | 190.7 ± 33.6 | 0.647 |
| HDL cholesterol, mg/dL | 62.3 ± 18.8 | 66.1 ± 17.0 | 0.503 |
| LDL cholesterol, mg/dL | 99.5 ± 25.8 | 103.9 ± 27.7 | 0.609 |
| Triglycerides, mg/dL | 119.3 ± 60.3 | 103.0 ± 42.7 | 0.324 |
| IL-6, pg/mL* | 3.3 ± 2.4 | 2.1 ± 1.0 | 0.016 |
| CRP, mg/L* | 1.9 ± 3.9 | 1.1 ± 1.9 | 0.076 |
| FMD, %* | 5.8 ± 1.9 | 8.1 ± 3.7 | 0.009 |
Values given as mean ± standard deviation, n (%), or median ± interquartile range.
Violated assumptions of parametric testing; median ± interquartile range are presented, and comparisons are from Mann-Whitney U tests.
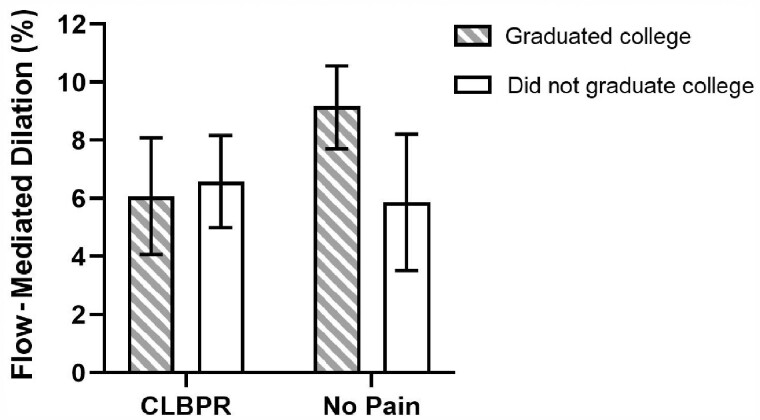
After controlling for educational level and BMI, none of the main effects or interactions with group membership were significant for the following cardiovascular health measures: systolic and diastolic blood pressure, cholesterol (total, HDL, and LDL), triglycerides, Il-6, and CRP. However, when endothelial function was examined, the group-by-education interaction term significantly explained 11.2% of the variance in FMD (P = 0.049). For older adults with no pain, being highly educated was associated with higher FMD values as compared with not being highly educated (mean ± standard error of 9.2 ± 0.7% vs. 5.9 ± 1.2%, respectively); see Figure 1. For older adults with CLBPR, however, FMD was low for both the highly and not highly educated (mean ± standard error of 6.1 ± 1.0% vs. 6.6 ± 0.8%, respectively).
Figure 1.
Group-by-education interaction for FMD. Means are adjusted for BMI.
Discussion
In our initial analyses without statistical adjustment, older adults with CLBPR had higher levels of low-grade systemic inflammation, as well as worse endothelial function. Furthermore, all current smokers were excluded from the study, and pack-years, a measure of past smoking exposure, was similar between groups, which indicates that our findings were independent of smoking status. However, it appears that educational level and BMI largely drove the initial between-group differences in pro-inflammatory marker (i.e., IL-6) concentration; after adjustment for those factors, the relationship between group membership and IL-6 was greatly attenuated and no longer statistically significant. Nevertheless, the between-group differences in endothelial function remained significant; these differences were moderated by educational level, such that those with higher educational level and no pain had better endothelial function than did the rest of the sample (i.e., all individuals with CLBPR and those with lower educational level and no pain) (Figure 1). Taken together, these results support our broader hypothesis that among older adults, individuals with CLBPR exhibit worse cardiovascular health than do those with no pain.
We theorized that the link between CLBPR and cardiovascular health outcomes could emerge from a similar pathogenesis of systemic inflammation. Inflammation sensitizes the nervous system, thereby increasing the risk of pain chronicity and elevating pain intensity [32], especially in those with a neuropathic pain component (e.g., those with CLBPR) [6]. Additionally, inflammation drives the development and progression of cardiovascular disease [7–9]. Although we found that CLBPR may be linked to higher levels of pro-inflammatory markers, these findings were attenuated after controlling for BMI and educational level. These findings are somewhat puzzling, given that the link between CLBPR and poor endothelial function (i.e., poor vascular health) remained significant after adjustment, but logical explanations exist. First, we chose to examine important markers of systemic inflammation, but they are by no means comprehensive: It is possible that other inflammatory pathways may contribute to these processes [32]. Second, it is possible that we were underpowered to detect group differences with regard to inflammation, despite having had sufficient power to detect differences in endothelial function. Third, although we used valid criteria for clinically classifying individuals as having CLBPR [20], misclassification was still possible; for example, hip osteoarthritis, which commonly co-occurs with low back pain, can mimic a clinical presentation similar to CLBPR.
Notwithstanding, this study is the first, to our knowledge, to examine the relationship between any chronic pain condition and endothelial function. Endothelial dysfunction is the initial step in the development of atherosclerosis and often precedes the development of other overt signs and symptoms of cardiovascular disease [23]. Prior work has shown that endothelial function is a predictor of cardiovascular events independent of age, gender, diabetes status, cigarette smoking, blood pressure, cholesterol, and baseline cardiovascular disease status [25]. Thus, our findings related to endothelial function are novel and important, especially when considering that traditional measures of cardiovascular health (i.e., blood pressure and cholesterol) did not differ between groups and would not indicate elevated cardiovascular risk for either group.
Educational level appears to have a unique impact on the relationship between group membership and endothelial function. Being more highly educated is associated with better endothelial function among older adults with no pain, indicating that education may have a protective effect, but that association appears to be absent for those with CLBPR. A recent study of a large sample of young, middle-aged, and older adults from Japan found that a cut point of 7.1% on FMD testing discriminated individuals who were and were not at risk for cardiovascular disease [33]. In our present study, we found that, on average, highly educated older adults without pain were above that cut point, indicating lower risk. In contrast, mean values fell below that threshold for older adults with CLBPR, regardless of educational level, and for pain-free participants without a college degree; taken altogether, these findings suggest that older adults with CLBPR may be at a uniquely higher risk of cardiovascular disease, comparable to that of older adults without a college education.
The pathway from pain to poor endothelial function may lie in how this condition affects physical activity levels. A hallmark sign of CLBPR is worsening symptoms with upright posture and walking, leading to significant mobility limitations [1, 2]. It is not surprising that prior studies have shown that individuals with CLBPR are significantly less physically active than their pain-free counterparts [34, 35]. Educational level is also linked to physical activity for older adults. Shaw et al. [36] found that higher levels of education stave off the natural decline of physical activity with age. Also, regular physical activity and exercise have been shown to attenuate the age-related decline in endothelial function [37]. In line with these findings, we found that being highly educated was associated with better endothelial function for older adults without pain. Interestingly, educational level was not linked to endothelial dysfunction in older adults with CLBPR, possibly indicating that the presence of CLBPR overrides the benefits of education on physical activity levels with aging. In future studies with larger samples and appropriate design considerations, it would be interesting to evaluate the role of physical activity as a mediator in the pathway from pain to endothelial dysfunction.
The findings from the present study have significant clinical relevance. Older adults with CLBPR appear to have worse cardiovascular health, independent of high BMI and smoking history. Thus, it appears that screening for these two traditional risk factors is not enough when evaluating cardiovascular disease risk for older adults with CLBPR. Furthermore, clinicians should consider the educational level of older adults with no pain when assessing cardiovascular health and disease risk; lower levels of education may predispose these individuals to higher risk. Finally, though clinicians should encourage all older adults to be physically active, it may be prudent to specifically target this at-risk subgroup of older adults with CLBPR, as well as older adults with lower educational levels who do not have CLBPR, with physical activity interventions to potentially improve, and prevent the decline of, cardiovascular health.
Some limitations should be noted for our study. First and foremost, our study was cross-sectional in nature, and thus, causal relationships cannot be determined. Second, though we found that older adults with CLBPR have worse cardiovascular health than their pain-free peers, our results were still somewhat modest from a statistical perspective. That said, these findings are to be expected to a certain degree; our goal was to isolate this painful condition from other factors (i.e., obesity and smoking) that could largely drive the relationship between it and poor cardiovascular health. Despite controlling for obesity and smoking, we were able to still detect a significant relationship, which is a strength of our study. Third, although we attempted to control for BMI through design considerations (i.e., exclusion of individuals with a BMI ≥35 kg/m2), our groups still differed; interestingly, the BMI of the CLBPR group was very much in alignment with previous studies of older adults with chronic low back pain [18, 19, 38]. We used a statistical adjustment to account for this limitation.
Conclusions
In conclusion, the relationship between CLBPR and cardiovascular health is complex and likely multifactorial. In this sample of currently nonsmoking older adults, CLBPR was linked to worse endothelial dysfunction. Furthermore, for older adults with no pain, being highly educated is associated with healthier endothelial function, indicating a potential protective effect on endothelial function; this effect does not appear to be present among those with CLBPR, who have lower endothelial function regardless of educational level.
Acknowledgments
The authors acknowledge Kenneth Kirschner for assisting with the inflammatory marker assays, as well as Michael Bryan, Bryce Muth, Danielle Kirkman, and Meghan Ramick for assisting with vascular function testing and analysis training.
Funding sources: This work was supported by the U.S. National Institutes of Health (grant numbers R01AG0412202, K12HD055931, and T32HD007490). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: None of the authors have any conflict of interest to disclose.
References
- 1. Lurie J, Tomkins-Lane C.. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hicks GE, Gaines JM, Shardell M, Simonsick EM.. Associations of back and leg pain with health status and functional capacity of older adults: Findings from the retirement community back pain study. Arthritis Rheum 2008;59(9):1306–13. [DOI] [PubMed] [Google Scholar]
- 3. Reid MC, Eccleston C, Pillemer K.. Management of chronic pain in older adults. BMJ 2015;350(2):h532–h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simon CB, Hicks GE.. Paradigm shift in geriatric low back pain management: Integrating influences, experiences, and consequences. Phys Ther 2018;98(5):434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Top 10 Causes of Death [Fact Sheet]: World Health Organization; 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed February 9, 2021).
- 6. Uher T, Bob P.. Neuropathic pain, depressive symptoms, and C-reactive protein in sciatica patients. Int J Neurosci 2013;123(3):204–8. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118(1):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: Moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007;49(21):2129–38. [DOI] [PubMed] [Google Scholar]
- 9. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 10. Korkiakoski A, Niinimäki J, Karppinen J, et al. Association of lumbar arterial stenosis with low back symptoms: A cross-sectional study using two-dimensional time-of-flight magnetic resonance angiography. Acta Radiol 2009;50(1):48–54. [DOI] [PubMed] [Google Scholar]
- 11. McBeth J, Symmons DP, Silman AJ, et al. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology 2008;48(1):74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiri R, Falah-Hassani K.. The effect of smoking on the risk of sciatica: A meta-analysis. Am J Med 2016;129(1):64–73.e20. [DOI] [PubMed] [Google Scholar]
- 13. Shiri R, Falah-Hassani K, Heliövaara M, et al. Risk factors for low back pain: A population-based longitudinal study. Arthritis Care Res 2019;71(2):290–9. [DOI] [PubMed] [Google Scholar]
- 14. Shiri R, Karppinen J, Leino-Arjas P, et al. Cardiovascular and lifestyle risk factors in lumbar radicular pain or clinically defined sciatica: A systematic review. Eur Spine J 2007;16(12):2043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiri R, Lallukka T, Karppinen J, Viikari-Juntura E.. Obesity as a risk factor for sciatica: A meta-analysis. Am J Epidemiol 2014;179(8):929–37. [DOI] [PubMed] [Google Scholar]
- 16. Schnohr P, Jensen JS, Scharling H, Nordestgaard BG.. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. Eur Heart J 2002;23(8):620–6. [DOI] [PubMed] [Google Scholar]
- 17. Wood RY, Giuliano KK, Bignell CU, Pritham WW.. Assessing cognitive ability in research: Use of MMSE with minority populations and elderly adults with low education levels. J Gerontol Nurs 2006;32(4):45–54. [DOI] [PubMed] [Google Scholar]
- 18. Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR.. The impact of chronic low back pain on older adults: A comparative study of patients and controls. Pain 2007;131(3):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hicks GE, Sions JM, Velasco TO, Manal TJ.. Trunk muscle training augmented with neuromuscular electrical stimulation appears to improve function in older adults with chronic low back pain: A randomized preliminary trial. Clin J Pain 2016;32(10):898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konno S, Kikuchi S, Tanaka Y, et al. A diagnostic support tool for lumbar spinal stenosis: A self-administered, self-reported history questionnaire. BMC Musculoskelet Disord 2007;8(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bray GA, Jablonski KA, Fujimoto WY, et al. , Diabetes Prevention Program Research Group. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr 2008;87(5):1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hicks GE, Manal TJ.. Psychometric properties of commonly used low back disability questionnaires: Are they useful for older adults with low back pain? Pain Med 2009;10(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deanfield JE, Halcox JP, Rabelink TJ.. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007;115(10):1285–95. [DOI] [PubMed] [Google Scholar]
- 24. Broxterman RM, Witman MA, Trinity JD, et al. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 2019;74(1):208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM.. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007;115(18):2390–7. [DOI] [PubMed] [Google Scholar]
- 26. Esper RJ, Nordaby RA, Vilariño JO, et al. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc Diabetol 2006;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Widlansky ME, Gokce N, Keaney JF, Vita JA.. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42(7):1149–60. [DOI] [PubMed] [Google Scholar]
- 28. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39(2):257–65. [DOI] [PubMed] [Google Scholar]
- 29. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300(1):H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 2017;158(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fairbank JC, Pynsent PB.. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–52. Discussion 52. [DOI] [PubMed] [Google Scholar]
- 32. Ellis A, Bennett DL.. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 2013;111(1):26–37. [DOI] [PubMed] [Google Scholar]
- 33. Maruhashi T, Kajikawa M, Kishimoto S, et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc 2020;9(2):e013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winter CC, Brandes M, Müller C, et al. Walking ability during daily life in patients with osteoarthritis of the knee or the hip and lumbar spinal stenosis: A cross sectional study. BMC Musculoskelet Disord 2010;11(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norden J, Smuck M, Sinha A, Hu R, Tomkins-Lane C.. Objective measurement of free-living physical activity (performance) in lumbar spinal stenosis: Are physical activity guidelines being met? Spine J 2017;17(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaw BA, Spokane LS.. Examining the association between education level and physical activity changes during early old age. J Aging Health 2008;20(7):767–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antunes BM, Rossi FE, Cholewa JM, Lira FS.. Regular physical activity and vascular aging. Curr Pharm Des 2016;22(24):3715–29. [DOI] [PubMed] [Google Scholar]
- 38. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2005;60(7):882–7. [DOI] [PubMed] [Google Scholar]