Abstract
Objective
Back pain is an elusive symptom complicated by a variety of possible causes, precipitating and maintaining factors, and consequences. Notably, the underlying pathology remains unknown in a significant number of cases. Changes to the intervertebral disc (IVD) have been associated with back pain, leading many to postulate that the IVD may be a direct source of pain, typically referred to as discogenic back pain. Yet despite decades of research into the neuroanatomy of the IVD, there is a lack of consensus in the literature as to the distribution and function of neural elements within the tissue. The current scoping review provides a comprehensive systematic overview of studies that document the topography, morphology, and immunoreactivity of neural elements within the IVD in humans.
Method
Articles were retrieved from six separate databases in a three-step systematic search and were independently evaluated by two reviewers.
Results
Three categories of neural elements were described within the IVD: perivascular nerves, sensory nerves independent of blood vessels, and mechanoreceptors. Nerves were consistently localized within the outer layers of the annulus fibrosus. Neural ingrowth into the inner annulus fibrosus and nucleus pulposus was found to occur only in degenerative and disease states.
Conclusion
While the pattern of innervation within the IVD is clear, the specific topographic arrangement and function of neural elements in the context of back pain remains unclear.
Keywords: Back Pain, Intervertebral Disc Degeneration, Peripheral Nervous System, Annulus Fibrosus, Nucleus Pulposus, Neuroanatomy
Introduction
Rationale
Back pain has a lifetime prevalence of 60–80% in the general population [1] and is the leading cause of years lived with disability worldwide [2]. In the United States, recent estimates of the total medical costs associated with the management of back pain range between $30 and $50 billion per annum [3]. Despite the societal burden of back pain and the demands it places on the healthcare system, a conclusive diagnosis of its underlying cause remains a challenge for clinicians. In fact, more than 85% of patients in the United States experience back pain for which an exact biological cause cannot be reliably identified [4–6].
In a minority of cases, back pain can be directly linked to clinical causes, including compression of adjacent nerve roots, traumatic injury to the spine, visceral conditions, infection, and inflammation of spinal tissues [7, 8]. Some of these causes of back pain may be reliably diagnosed and treated, such as anatomical deformities, tumors, infection-induced abscesses, and fractures of the spine that compress nerves or damage surrounding soft tissues [8]. However, back pain is also associated with degenerative changes to the intervertebral disc (IVD), which has led some to postulate that the disc itself may be a source of pain, termed discogenic back pain [2, 7–10].
The IVD is a fibrocartilaginous structure composed of three distinct components. The central nucleus pulposus is rich in proteoglycans and water, creating a high intradiscal pressure that enables the IVD to resist compressive forces [11]. The peripheral annulus fibrosus functions to stabilize and dissipate tensile forces through ∼20 concentric collagen lamellae [12]. The vertebral endplates are formed by two regions: a hyaline cartilage region and a porous bone region that connects to the vertebral bone on both the superior and inferior aspects of the IVD. The endplate serves both to anchor the IVD to the vertebra and acts as a selectively permeable barrier for nutrient diffusion [12].
The IVD is often referred to as the largest avascular tissue of the body also lacking direct innervation. A recent review by our group summarized the available literature characterizing vascularization of the IVD over the lifespan and highlighted changes associated with age and state of degeneration or damage [13]. This review of the literature underscored the poorly understood relationship between neural and vascular ingrowth in the IVD, particular in the context of degeneration. Age, genetic susceptibility, exposure to chronic mechanical stress, and gradual depletion of nutrients can lead to widespread cellular disturbance and accelerated degeneration of the IVD [9, 10]. Modifications of the extracellular matrix within the IVD resulting from matrix catabolism or altered matrix synthesis, together with increased intradiscal expression of proinflammatory cytokines and neurotrophins, can regulate pathological nerve ingrowth into the IVD that results in the synthesis of pain-related neuropeptides [9, 14, 15]. As such, it is known that neural and vascular ingrowth into damaged or degenerated IVD tissues may enable discogenic back pain.
Despite extensive investigation into the pathophysiology that might underly discogenic back pain, the precise molecular mechanisms by which age-related alterations of IVD tissues disrupt or sensitize nearby neural structures, or promote neoinnervation, remain unclear. In addition, the topographical organization and type of intradiscal neural elements within the annulus fibrosus, nucleus pulposus, and vertebral endplates are poorly characterized in both healthy and degenerated IVDs. To this end, the current scoping review provides a comprehensive, systematic evaluation of currently available literature investigating the presence of neural elements within human IVDs across the lifespan.
Research Questions
What neural elements are present in the human IVD across the lifespan, and how are they organized within the annulus fibrosus, nucleus pulposus, and vertebral endplates?
The review question was designed to generate a comprehensive list of articles that investigated the presence of neural elements within the human IVD. An a priori search of the current literature was conducted (PubMed, BMJ Open, Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports: May 2020) and no published or ongoing scoping or systematic reviews on the topic were identified. After preliminary evaluation of the search results, the authors defined three additional sub-questions: (i) is there a relationship between the location and/or type of neural elements present within the IVD and the state of IVD degeneration? (ii) do neural elements within the IVD always accompany vascular structures? (iii) which methods are the most effective to study neural elements in the IVD? Together, the primary aim of the current scoping review was to document the topographical arrangement of neural elements within healthy and degenerated human IVDs across the lifespan.
Methods
The scoping review was designed according to the Preferred Reporting Items for Systematic Reviews Extension for Scoping Reviews (PRISMA-ScR) checklist [16]. Systematic reviews are designed to answer detailed questions that are focused on a specific topic, whereas scoping reviews target broader research questions. The 20-item checklist used herein applies the PRISMA-ScR guidelines to delineate specific aspects of knowledge synthesis that are specific to scoping reviews [16].
Eligibility Criteria and Information Sources
The aim of the scoping review was to assess the topographical arrangement and types of neural elements present in the IVD across the human lifespan. To be included in the analysis, articles had to describe the presence and/or characteristics of neural elements within human IVDs (e.g., spatial localization, changes with age, types of neural elements). Only peer-reviewed research articles were included, with the exception of one letter to the editor which contained significant details on study population, methods, and results [17]. Review articles, abstracts, commentaries, and articles printed in a non-English language were excluded. Review articles were screened for citations of primary research reports eligible for inclusion.
Human Criteria
No restrictions were placed upon the age, sex, ethnicity, or health-status (non-spine affecting pathologies—e.g., cardiovascular disease, obesity, diabetes) of tissues assessed in the included studies. Studies that used animal models or interrogated molecular interactions with cultured IVD cells or neurons were excluded.
IVD Criteria
Studies examining both non-degenerate and degenerate IVD tissues were included to reflect the aging process [18], whereas herniated, ankylotic, or any otherwise diseased IVDs were excluded. However, many studies utilized experimental groups of mixed etiology (i.e., containing a combination of degenerate IVDs with a history of other spine-related pathologies). In these cases, all tissues were included for analysis in the scoping review, as studies often did not denote a specific health history for each donor.
Neural Element Criteria
Neural elements of all types were included and ranged from specialized nerve endings to myelinated axons (Supplementary Data 1). In addition, the scoping review included studies that investigated neural elements using multiple methodologies, including gross dissection, histology, and medical imaging. Articles focused on describing the neural elements of the osseous and ligamentous features of the spine were excluded if they did not also describe the neural elements of the IVD.
Articles were retrieved from six primary information sources: Scopus (1966–), MEDLINE (1946–), EMBASE (1947–), PubMed (1966–), Web of Science (1900–), and BIOSIS Previews (1926–). These databases provide a broad range of literature in the biological and physical sciences. The most recent search was completed through the Web of Science database on May 1, 2020. Following analyses and prior to submission, the systematic search was re-run using each information source on December 21, 2020, to ensure any pertinent articles published during the review process were captured.
Search Strategy
The authors implemented a systematic three-step search strategy in line with the framework developed by the Joanna Briggs Institute [19]. This process began with an initial search in PubMed to find seminal articles relevant to the three components of the research question: IVDs, humans, and neural elements. This search was then repeated in two information sources (Medline and Embase) to index novel terms or alternative spellings in article titles and/or abstracts that had not been captured. This iteration was then reviewed by an institutional librarian and an expert in neuroanatomy to ensure that the search strategy was organized appropriately and contained a comprehensive set of keywords surveying the seminal aspects of the research question. Finally, the precise syntax of the search strategy was modified such that it could be applied to each information source. An example of a comprehensive search strategy for one of the information sources can be found in Supplementary Data 1. The reference lists of all full-text articles that met the criteria for inclusion were reviewed in a final step to retrieve any relevant articles that may have been missed by the search strategy.
Selection of Sources of Evidence
Articles obtained during the search of six separate databases were compiled and imported into Mendeley (Version 1.19.4, Elsevier Inc., New York, NY) to organize and remove duplicates before sending to a second reviewer. Both reviewers independently screened titles and abstracts. This was followed by a consensus meeting to discuss and resolve any disagreements. The resolved disagreements were the exclusion of reviews, articles focused on molecular interactions with cultured IVD cells or neurons, and articles that describe neural elements in the spine and surrounding tissues but not the IVD. Next, eligible full-text articles were retrieved and independently screened by each reviewer, followed by another consensus meeting to resolve disagreements. The inter-rater reliability associated with analysis of both titles/abstracts and full-text articles was calculated using Cohen’s kappa [20] with SPSS Statistics (Version 26.0, IBM Corp., Armonk, NY). This statistical coefficient represents the measure of agreement between raters for nominal scales.
Data Charting Process
Data were charted from the final list of full-text articles by a single reviewer. Before data were charted from all included articles, the chart was tested by randomly selecting two articles and each reviewer independently charting the appropriate data and these results were compared and discussed. Throughout the data charting by the lead reviewer (AMRG), the chart was modified in an iterative manner to ensure accurate documentation of study data. The data items charted included citation information, study sample characteristics, methods of neural element visualization, and pertinent results concerning the topography and type of neural elements within the IVD.
Synthesis of Results
Results from the process of data charting were tabulated and organized by characteristics of the study sample and the neural elements within the IVD tissues. Data were further categorized by age and health status, where possible.
Results
Selection of Sources of Evidence
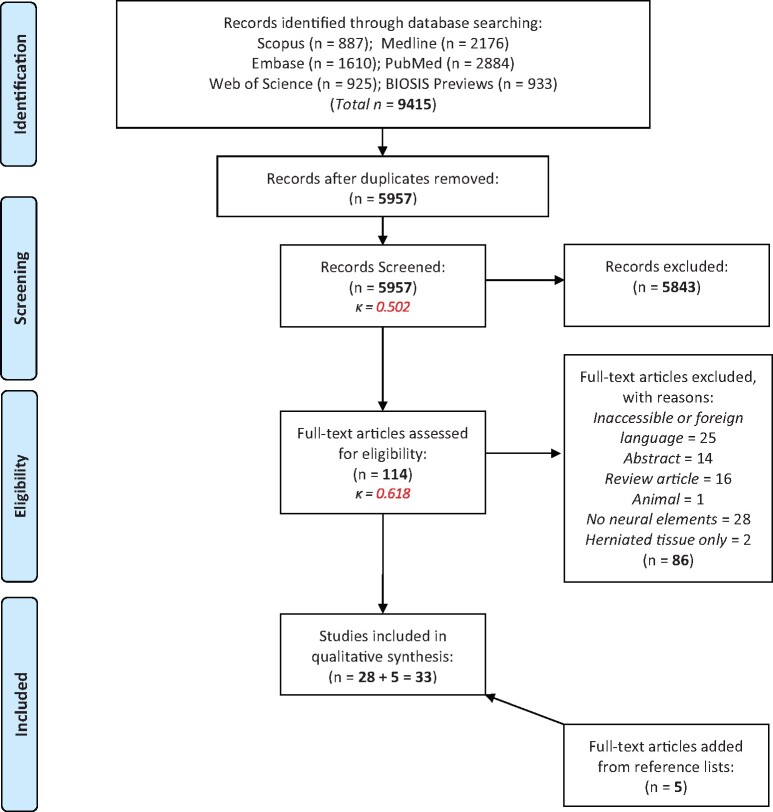
The PRISMA-ScR flow diagram [16] (Figure 1) depicts each step of the article selection process, including the specific rationale for exclusion of full-text articles. A total of 9,415 articles were retrieved following the six-database search. After removal of duplicate articles, 5,957 citations were evaluated by two independent reviewers in a two-step screening process. First, screening of titles/abstracts for key concepts such as the use of human tissue, study of the IVD, and data of neural elements within the IVD, resulted in the exclusion of 5,843 articles. The remaining 114 articles proceeded to full-text screening, which eliminated 86 articles (e.g., non-English language, lacking description of neural elements). The remaining 28 articles were included for final examination. Inter-rater reliability analysis revealed kappa values of 0.50 and 0.62 for title/abstract and full-text screening, respectively. Landis and Koch suggest that Kappa values of 0.41 ≤ 0.60 and 0.61 ≤ 0.80 indicate moderate to substantial agreement between independent reviewers, respectively [21]. Finally, review of the reference lists of the 28 full-text articles included for final examination led to the inclusion of an additional four articles in the final analyses. Thus, a total of 33 articles met the eligibility criteria for the current scoping review.
Figure 1.
PRISMA flow diagram of the article selection process of information sources.
Results of Individual Sources of Evidence
Citation information and study sample characteristics for each full-text article included in the final analysis is documented in Table 1. Included articles were published between 1940 and 2019 and included contributions from around the world.
Table 1.
Article citation information and study population characteristics
| Citation Information |
Study Population |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Location | Study Design | Tissue | Region | Donors | Female | Male | Age | Health Status |
| Roofe [22] | 1940 | USA | Descriptive | pm | L4–5 | 7 | 1 | 6 | 2, 50–67 | … |
| Wiberg [23] | 1949 | SWE | Descriptive | s | … | 200 | … | … | … | … |
| pm | Lumbosacral | … | … | … | … | …… | ||||
| pm | Lumbar IVDs | … | … | … | fetal, adult | … | ||||
| Pedersen [24] | 1956 | USA | Descriptive | pm | Lumbosacral | 6 | … | … | fetal, adult | … |
| Malinsky [25] | 1959 | CZE | Descriptive | pm | Full lumbar spine | 9 | … | … | fetal | … |
| pm, s | L4–5 | 13 | … | … | 0–19 | … | ||||
| s | L4–5 | 14 | … | … | 20–77 | … | ||||
| Ferlic [26] | 1963 | USA | Descriptive | s | C5–6 | 1 | … | … | … | IVD degeneration |
| pm | C3–T1 | 6 | … | … | fetal, adult | … | ||||
| Jackson [27] | 1966 | USA | Descriptive | s | L4–5 | 15 | … | … | … | IVD degeneration |
| pm | Lumbar or L4–5 | 21 | … | … | fetal, adult | … | ||||
| Yoshizawa [28] | 1980 | JPN | Case-control | s | Lower lumbar | 17 | … | … | … | LBP |
| s | Lumbar | 4 | … | … | … | Similar problems (i.e., LBP) | ||||
| pm | … | … | … | … | adult | “normal IVDs” | ||||
| Bogduk [29] | 1981 | AUS | Descriptive | pm | Lumbar | 4 | … | … | … | … |
| Bogduk [30] | 1988 | AUS | Case-control | s | Cervical | 3 | … | … | … | Spinal fusion surgery |
| pm | Cervical | 5 | … | … | adult | … | ||||
| Coppes [17] | 1990 | NLD | Case-control | s | L4–5 | 10 | … | … | … | IVD degeneration, LBP |
| c | L5–S1 | 2 | … | … | … | Spinal tumor surgery | ||||
| Mendel [31] | 1992 | USA | Descriptive | pm | Cervical | 4 | … | … | adult | … |
| Ashton [32] | 1994 | GBR | Descriptive | s | L4–S1 | 12 | 5 | 7 | 29–50 | Back pain |
| Roberts [33] | 1995 | GBR | Descriptive | s | Thoracic-lumbar (not specified) | 14 | … | … | 18–51 | LBP |
| s | 15 | … | … | 5–53 | Scoliosis | |||||
| pm | 1 | … | … | 49 | … | |||||
| Brown [34] | 1997 | GBR | Case-control | s | L3–S1 | 15 | 11 | 4 | 17–62 | IVD degeneration, LBP |
| pm | “similar” | 7 | … | … | 53–69 | No chronic back pain | ||||
| Coppes [35] | 1997 | NLD | Case-control | s | L3–S1 | 10 | 6 | 4 | 24–51 | IVD degeneration, LBP |
| c | L3–L5 | 2 | 1 | 1 | 29, 51 | Spinal tumor surgery | ||||
| Freemont [36] | 1997 | GBR | Case-control | s | L3–5 | 38 | … | … | 20–59 | Chronic back pain (split into pain and non-pain levels) |
| pm | L3–5 | 31 | … | … | matched | No spinal disorders | ||||
| Palmgren [37] | 1999 | FIN | Descriptive | pm | L1–S1 | 5 | 4 | 1 | 13–53 | Non-degenerated IVDs |
| Johnson [38] | 2001 | GBR | Descriptive | s | L2–3; L4–S1 | 15 | 8 | 7 | 14–50 | Injury, IVD degeneration, LBP |
| Bucknill [39] | 2002 | GBR | Descriptive | s | Lumbar | 40 | 22 | 18 | 20–78 | IVD degeneration or prolapse |
| Freemont [40] | 2002 | GBR | Case-control | s | L3–S1 | 36 | 9 | 12 | 23–58 | IVD degeneration, pain |
| s | 8 | 12 | 26-54 | IVD degeneration, no pain | ||||||
| pm | … | 12 | 4 | 8 | 22–59 | “normal IVD” | ||||
| Peng [41] | 2005 | CHN | Case-control | s | Lumbar | 17 | 5 | 12 | 19–55 | LBP |
| c | Lumbar | 12 | … | … | 29–61 | No LBP, “normal” | ||||
| pm | L4–S1 | 5 | … | … | 22–45 | No spinal disorders | ||||
| Ohtori [42] | 2006 | JPN | Case-control | s | L4–S1 | 14 | 6 | 8 | 20–46 | IVD degeneration or herniation, LBP |
| pm | L1–2; 4–5 | 4 | 2 | 2 | 13–30 | Scoliosis, fracture | ||||
| Ozawa [43] | 2006 | JPN | Descriptive | s | L4–5 or L5–S1 | 8 | 4 | 4 | 21–45 | IVD degeneration or herniation, LBP |
| Johnson [44] | 2007 | GBR | Case-control | s | Lumbar | 21 | 15 | 8 | 11–62 | IVD degeneration or prolapse, LBP |
| c | Lumbar | 2 | 21, 21 | Fracture, dislocation | ||||||
| Kokubo [45] | 2008 | JPN | Case-control | s | Cervical | 198 | 77 | 121 | 25–78 | IVD herniation, pain |
| s | Cervical | 166 | 61 | 105 | 32–83 | Spondylosis, pain | ||||
| pm | Cervical | 4 | … | … | 68–78 | No spinal disorders | ||||
| Dimitroulias [46] | 2010 | GRC | Descriptive | pm | L4–S1 | 15 | 7 | 8 | 15–66 | No spinal disorders |
| Martins [47] | 2010 | BRA | Descriptive | pm | L1–L5 | 10 | 5 | 5 | 30–85 | No spinal disorders |
| Fields [48] | 2014 | USA | Descriptive | pm | T11–S1 | 7 | 2 | 5 | 51–67 | Endplate pathologies |
| Binch [49] | 2015 | GBR | Case-control | s | C3–4, 5–7; L2–S1 | 61 | … | … | … | Nerve root compression |
| pm | L3–S1 | 22 | … | … | … | … | ||||
| Yang [50] | 2017 | CHN | Case-control | s | C3–T1 | 54 | 24 | 30 | 55 ± 11 | Cervical spondylosis with vertigo |
| c | C3–T1 | 62 | 25 | 37 | 54 ± 9 | Cervical spondylosis without vertigo | ||||
| pm | C3–T1 | 8 | 3 | 5 | 52 ± 9 | No spinal disorders | ||||
| Lama [51] | 2018 | CAN | Case-control | s | L2–S1 | 10 | 8 | 2 | 37–68 | IVD herniation (sciatica) |
| s | L3–S1 | 11 | 5 | 6 | 35–74 | IVD herniation (no sciatica) | ||||
| s | L2–S1 | 11 | 6 | 5 | 39–72 | IVD degeneration | ||||
| s | T12–L5 | 8 | 8 | 0 | 14–15 | Scoliosis | ||||
| Yang [52] | 2018 | CHN | Case-control | s | C3–T1 | 52 | 23 | 29 | 50 ± 8 | Transient neck pain and dizziness |
| c | C3–7 | 6 | 2 | 4 | 49 ± 14 | Acute spinal cord injury | ||||
| pm | C3–T1 | 8 | 3 | 5 | 52 ± 9 | No spinal disorders | ||||
| Wu [53] | 2019 | CHN | Case-control | s | C3–7 | 34 | 13 | 21 | 49 ± 10 | Chronic neck pain |
| c | C3–7 | 36 | 16 | 20 | 47 ± 10 | Symptom control: mild or no neck pain | ||||
| pm | C3–7 | 8 | 3 | 5 | 52 ± 9 | Histology control: no spinal disorders | ||||
Citation information. First author is listed. Year of publication is displayed. Location listed as 3-letter country abbreviation, based on the corresponding author’s information.
Study population. Tissue sources: s = surgery; c = controls; and pm = post-mortem. Region of the spine: C = cervical; T = thoracic; L = lumbar; and S = sacral. The age range studied is displayed in years. Select authors only presented age as means ± standard deviation.
“…” represents insufficient information provided.
IVD=intervertebral disc; LBP=low back pain.
Study Design and Source of Tissue
The included articles implemented either descriptive (17/33) or case-control (16/33) study designs to evaluate IVDs tissues obtained from two primary sources: post-mortem and surgical donors (Table 1). Post-mortem evaluation of autopsy or embalmed cadaveric material enabled analysis of the intact IVD. Tissues retrieved from surgery were limited to biopsies or en bloc resection (most often of the anterior aspect of the IVD).
In the descriptive studies, IVD tissues were obtained from spine-related surgical procedures (4/17), post-mortem tissues (8/17), or from a combination of both (5/17).
In the case-control studies, all surgical samples were excised during procedures to alleviate chronic back pain, such as interbody fusions, nerve root decompressions, and discectomies. Specific IVDs of patients with spine-related disease or reported back pain were selected following discography. Tissues excised from surgery were regularly compiled into one experimental group of mixed etiology (13/16). Few studies organized surgical tissues based on the presence, or absence, of a particular symptom (e.g., pain or no pain) or spine-related disease (e.g., scoliosis). IVDs included in control groups typically originated from post-mortem samples only (8/16), surgical samples only (4/16), or from post-mortem and surgical samples (4/16). In the latter case, surgical control samples were taken from spinal regions that lacked patient-reported symptom or spine-related disease. There was tremendous variability in the reporting of control IVD tissues. Most control groups listed age ranges and sex but were not identified as age- or sex-matched to the case group (11/16). One control group was matched for age but not sex (1/16), and the remaining studies presented minimal (i.e., used nondiscriminatory age brackets such as adult or fetal) or no demographic data (4/16).
Anatomical Region
IVDs were most often obtained from the lumbosacral spine (16/33). Some studies focused solely on the cervical region (7/33), some examined tissues from two or more anatomical regions (3/33), and the remaining studies did not report the anatomical region from which IVDs were retrieved (7/33). It is worth noting that we considered IVDs between C7/T1 to be part of the cervical region in the aforementioned groupings. Most studies included IVD tissues from multiple vertebral levels, rather than focusing on a single motion segment (i.e., L1–L5, not just L1/L2).
Number of Donors, Distribution of Sex, and Age
Generally, studies focused on neural elements of the human IVD were limited to relatively small sample sizes (overall mean = 39 ± 66, median = 16.5, range = 3 to 368).
Many articles did not include information on the sex of donors used in their studies (12/33). In the studies that did report sex, both female and male donors were included (21/33). Of these, some included more females (7/21), more males (11/21), or an equal distribution of females and males (3/21). Results for each group were always pooled (irrespective of sex) by the study authors, and details about the neural elements were described for the group as a whole. Therefore, the relationship between sex and the presence of neural elements in the IVD could not be examined in the current scoping review.
The age of donors ranged from fetal and infantile tissues to older adults over 80 years of age. However, the majority of studies only reported the age range for the sake of describing the study sample. Data concerning the neural elements within the IVD were never directly stratified by age. In the current scoping review, data were organized by age when possible. In cases where age was not clearly reported, results were not assumed to reflect any particular age group and instead were presented across all ages.
Donor Health Status
The health history of donors included in descriptive reports are organized into three groups: (i) no health history listed, (ii) tissues obtained from donors without symptoms of back pain, and (iii) tissues obtained from donors with back pain due to degeneration or other forms of IVD disease.
In case-control studies, pathological IVD tissues were often reported as pain producing (determined by discography). In some cases, herniated and/or spondylotic tissues were grouped together with degenerated tissues. For this reason, the current scoping review could not draw firm conclusions about the relationship between IVD innervation and age-related degeneration from all included articles.
Most control tissues were obtained from post-mortem donors (either cadaveric or from autopsy) with no IVD herniation or degeneration. However, select studies retrieved IVDs from surgical procedures for scoliosis, vertebral fractures, or spinal tumors. Thus, these control groups did not necessarily reflect “healthy” control IVDs.
Study Methodologies
Articles used histological or immunohistochemical techniques to investigate the neural elements within the IVD. Some studies combined histological techniques with other imaging modalities (4/33), gross dissection (5/33), or both (1/33). For histological examination, standard tissue fixation was used to preserve IVD tissues (i.e., alcohol, paraformaldehyde, formalin, methanol, or acetone). Tissues were paraffin-embedded and sectioned using a microtome or embedded in optimal cutting temperature compound and sectioned using a cryostat. In either case, IVD tissue was typically sectioned at a thickness of 30 microns. Standard immunohistochemical labeling methods were followed with all antisera listed in Table 2; counter-staining was used to visualize other tissue components of the IVD. In older studies that did not employ immunostaining, neural elements were visualized using a variety of silver impregnation techniques, gold chloride, or osmium tetroxide staining. While most reports used donor health history and symptomatology to assign IVD samples into case or control groups (28/33), others used more standardized classification systems based upon histological or MRI grading to determine the level of IVD degeneration (5/33) [38, 44, 47, 48, 51]. Gross dissection was used in two studies to trace the neuroanatomical extent of fibers traveling to the IVD [29, 30].
Table 2.
List of markers used to identify neural elements in the human intervertebral disc
| Abbreviation | Full Name(s) | Function | Location |
|---|---|---|---|
| AChE [17, 35] |
|
|
|
| CGRP [32–34, 43] |
|
||
| GAP43 [36, 40, 45] |
|
|
|
| GFAP [38] |
|
|
|
| IB4 [43] |
|
|
|
|
NF68 [45] |
|
|
|
| NGF [45] |
|
|
|
| NPY/CPON [32–34, 37] |
|
|
|
| p75 [40] |
|
|
|
| PGP9.5 [32–34, 36–40, 42, 43, 48, 49, 51] |
|
|
|
| PRPH [38] |
|
|
|
| PTN [44] |
|
|
|
| S100 [46, 47, 50, 52] |
|
|
|
| SNS/PN3 and NaN/SNS2 [39] |
|
|
|
| SP [17, 32–37, 41, 45, 51–53] |
|
||
| SYN [37] |
|
|
|
| TrkA [40] |
|
|
|
| VIP [32, 33, 41] |
|
Massoulie et al. 1994. Prog. Neurobiol. 41(1):31–91. doi: 10.1016/0301-0082(93)90040-y.
Chen et al. 2010. J. Clin. Neurosci. 17(1):87–91. doi: 10.1016/j.jocn.2009.03.042.
McCulloch et al. 1986. Proc. Natl. Acad. Sci. USA. 83(15):5731–5. doi: 10.1073/pnas.83.15.5731.
Benowitz and Routtenberg, 1997. Trends. Neurosci. 20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2.
Curtis et al. 1992. J. Cell. Biol. 116(6):1455–64. doi: 10.1083/jcb.116.6.1455.
Middeldorp, 2001. Prog. Neurobiol. 93(3):421–43. doi: 10.1016/j.pneurobio.2011.01.005.
Roessman et al. 1980. Brain. Res. 200(1):13–21. doi: 10.1016/0006-8993(80)91090-2.
Davidoff et al. 2002. Acta. Histochem. 104(1):39–49. doi: 10.1078/0065-1281-00630.
Bennett et al. 1998. J. Neurosci. 18(8):3059–72. doi: 10.1523/JNEUROSCI.18-08-03059.1998.
Khalil et al. 2018. Nat. Rev. Neurol. 14(10):577–89. doi: 10.1038/s41582-018-0058-z.
Laser-Azogui et al. 2015. Curr. Opin. Cell. Biol. 32:92–101. doi: 10.1016/j.ceb.2015.01.003.
Denk et al. 2017. Annu. Rev. Neurosci. 40:307–25. doi: 10.1146/annurev-neuro-072116-031121.
Crowder and Freeman, 1998. J. Neurosci. 18(8):2933–43. doi: 10.1523/JNEUROSCI.18-08-02933.1998.
Decressac and Barker, 2012. Exp. Neurol. 238(2):265–72. doi: 10.1016/j.expneurol.2012.09.004.
Kuo et al. 2007. Nat. Med. 13(7):803–11. doi: 10.1038/nm1611.
Benito-Gutierrez et al. 2006. Mol. Cell. Neurosci. 31(2):179–92. doi: 10.1016/j.mcn.2005.09.007.
Doran et al. 1983. J. Neurochem. 40(6):1542–7. doi: 10.1111/j.1471-4159.1983.tb08124.x.
Hol and Capetanaki, 2017. Cold. Spring. Harb. Perspect. Biol. 9(12):a021642. doi: 10.1101/cshperspect.a021642.
Jin et al. 2009. Neurosurg. Rev. 32(4):387–93. doi: 10.1007/s10143-009-0202-8.
Marenholz et al. 2004. Biochem. Biophys. Res. Commun. 322(4):1111–22. doi: 10.1016/j.bbrc.2004.07.096.
Tate et al. 1998. Nat. Neurosci. 1(8):653–5. doi: 10.1038/3652.
Dib-Hajj et al. 1998. Proc. Natl. Acad. Sci. USA. 95(15):8963–8. doi: 10.1073/pnas.95.15.8963.
Zieglgansberger, 2018. Cell. Tissue. Res. 375(1):227–41. doi: 10.1007/s00441-018–2922-y.
Mashagi et al. 2016. Cell. Mol. Life. Sci. 73(22):4249–64. doi: 10.1007/s00018-016–2293-z.
Wiedenmann et al. 1986. Proc. Natl. Acad. Sci. USA. 83(10):3500–4. doi: 10.1073/pnas.83.10.3500.
Iwasaki et al. 2019. F1000Res. 8: F1000 Faculty Rev-1629. doi: 10.12688/f1000research.18039.1.
Synthesis of Results
An assortment of neural elements were identified within the IVD and categorized as: (i) sensory nerve fibers that were unassociated with blood vessels of variable thickness and immunoreactivity; (ii) perivascular nerve fibers; or (iii) mechanoreceptors. Neural elements were identified by qualitative observation of nerve morphology or immunostaining. Table 2 presents the list of all the antibodies used to analyze neural elements within the IVD.
Annulus Fibrosus
The annulus fibrosus was the most innervated tissue of the IVD, as neural elements were described in nearly all of the included articles (32/33, Table 3), except for one report [24]. The general trend was that neural elements were localized to the outer one-third of the annulus fibrosus in control IVDs and that these elements infiltrated into the inner two-thirds of the annulus fibrosus in pathological IVDs with a history of back pain, degeneration, or related spinal pathologies. Although specimen age was not directly correlated to IVD innervation in individual studies, cross-referencing IVD innervation data with specimen age ranges across all studies revealed that nerve endings may be more abundant and morphologically complex across the lifespan (Figure 2).
Table 3.
Neural elements in the annulus fibrosus
| First Author | Year | Neural Markers and Stains | IVD Health Status | Description of Neural Elements |
|---|---|---|---|---|
| Roofe [22] | 1940 | Silver stain; gold chloride | … |
|
| Wiberg [23] | 1949 | Silver stain | … |
|
| Pedersen [24] | 1956 | Silver stain; Masson’s stain | … |
|
| Malinsky [25] | 1959 | Silver stain | … |
|
| Ferlic [26] | 1963 | Silver stain |
IVD degeneration … |
|
| Jackson [27] | 1966 | Cholinesterase; silver stain |
IVD degeneration … |
|
| Yoshizawa [28] | 1980 | Cholinesterase; silver stain |
LBP Similar problems (i.e., LBP) Normal IVD (control) |
|
| Bogduk [29] | 1981 | Silver stain; osmium tetroxide | … |
|
| Bogduk [30] | 1988 | Silver stain; cholinesterase |
Spinal fusion surgery … |
|
| Coppes [17] | 1990 | AChE; NF; SP |
IVD degeneration, LBP Spinal tumor surgery (control) |
|
| Mendel [31] | 1992 | Gold chloride | … |
|
| Ashton [32] | 1994 | PGP9.5; CGRP; SP; VIP; CPON | Back pain |
|
| Roberts [33] | 1995 | PGP9.5; CGRP; SP; VIP; NPY; Mayer’s hematoxylin |
LBP Scoliosis (control) Not listed |
|
| Brown [34] | 1997 | PGP9.5; CGRP; SP; NPY; H&E; toluidine blue |
IVD degeneration, LBP No chronic back pain (control) |
|
| Coppes [35] | 1997 | AChE; NF97; SP |
IVD degeneration, LBP Spinal tumor surgery (control) |
|
| Freemont [36] | 1997 | PGP9.5; SP; GAP43; H&E |
Chronic back pain (split into non-pain and pain level subgroups) No spinal disorders (control) |
|
| Palmgren [37] | 1999 | SYN; PGP9.5; CPON; SP; hematoxylin | Non-degenerated IVDs |
|
| Johnson [38] | 2001 | PGP9.5; NF200; peripherin; GFAP; H&E |
Injury, IVD degeneration, LBP [all specimens allocated degeneration score] |
|
| Bucknill [39] | 2002 | PGP9.5; NF; SNS/PN3; NaN/SNS2; neutral red | IVD degeneration or prolapse |
|
| Freemont [40] | 2002 | PGP9.5; p75; GAP43; TrkA; toluidine blue |
IVD degeneration, pain IVD degeneration, no pain Normal IVD (control) |
|
| Peng [41] | 2005 | NF200; SP; VIP; hematoxylin |
LBP No LBP No spinal disorders (control) |
|
| Ohtori [42] | 2006 | PGP9.5 |
IVD degeneration or herniation, LBP Scoliosis, fracture |
… |
| Ozawa [43] | 2006 | PGP9.5; CGRP; IB4 | IVD degeneration or herniation, LBP |
|
| Johnson [44] | 2007 | NF200; pleiotrophin; Mayer’s hematoxylin |
IVD degeneration or prolapse, LBP Fracture, dislocation (control) [all specimens allocated degeneration score] |
|
| Kokubo [45] | 2008 | NF68; GAP43; SP; NGF; H&E |
IVD herniation, pain Spondylosis, pain No spinal disorders (control) |
|
| Dimitroulias [46] | 2010 | S100; Harris hematoxylin | No spinal disorders |
|
| Martins [47] | 2010 | S100; hematoxylin |
No spinal disorders [all specimens allocated degeneration score] |
|
| Fields [48] | 2014 | PGP9.5; Heidenhain stain |
Endplate pathologies [all specimens allocated degeneration score] |
|
| Binch [49] | 2015 | NF200; PGP9.5; H&E |
Nerve root compression Not listed (control) |
|
| Yang [50] | 2017 |
S100; NF200 Harris hematoxylin |
Cervical spondylosis with vertigo Cervical spondylosis without vertigo No spinal disorders (control) |
|
| Lama [51] | 2018 | PGP9.5; SP; H&E |
IVD herniation (sciatica) IVD herniation (no sciatica) IVD degeneration Scoliosis (control) [all specimens allocated degeneration score] |
|
| Yang [52] | 2018 | S100; SP; H&E |
Transient neck pain and dizziness Acute spinal cord injury No spinal disorders (control) |
|
| Wu [53] | 2019 | SP; H&E |
Chronic neck pain Mild or no neck pain No spinal disorders (control) |
|
“…” represents insufficient information provided.
IVD=intervertebral disc; AF=annulus fibrosus; LBP=low back pain; H&E=hematoxylin and eosin; full names of neural marker acronyms, alongside functional descriptions, can be found in Table 2.
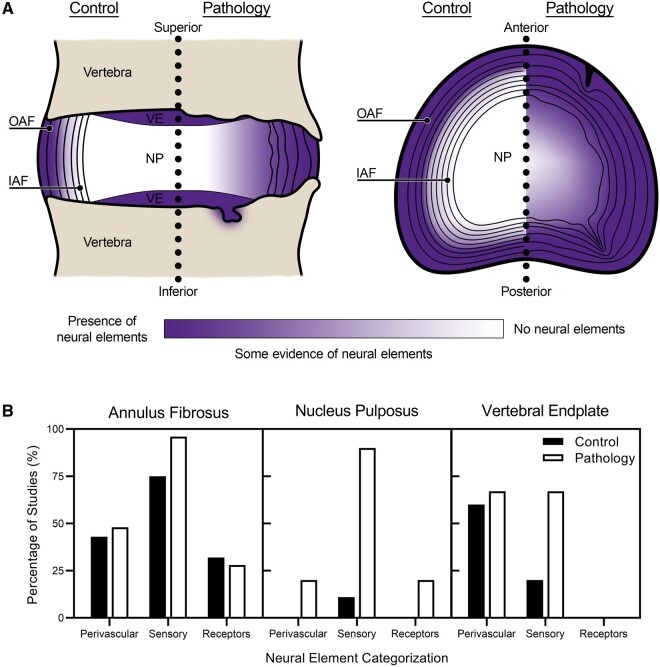
Figure 2.
Summary of neural elements reported within the human intervertebral disc. (A) Frontal section of a motion segment of the vertebral column (left) and a transverse view of the intervertebral disc (right). The localization and prevalence of neural elements, as reported by the authors of the studies analyzed in the current scoping review, is displayed using a scale that ranges from purple (evidence of neural elements) to white (no evidence of neural elements). Illustration is organized as control compared to pathology (i.e., chronic pain, degeneration, structural damage, etc.). Schematic and anatomical features are not drawn to scale. OAF, outer annulus fibrosus; IAF, inner annulus fibrosus; NP, nucleus pulposus; and VE, vertebral endplate. (B) The percentage of studies included in the scoping review that documented the presence of neural elements (perivascular, sensory or mechanoreceptors) in the annulus fibrosus, nucleus pulposus and vertebral endplate of the intervertebral disc.
Nerve fibers without adjacent blood vessels, and likely sensory in nature, were found throughout the outer and inner annulus fibrosus in the vast majority of studies [17, 22, 25–41, 43–53]. These fibers were typically described as free nerve endings and coursed both obliquely and in parallel to the circumferential lamellae of the annulus fibrosus. Neural infiltration into the inner annulus fibrosus was more frequent in painful IVD tissues or within fissures or damaged regions of the annulus fibrosus than in controls or IVDs with no listed spinal pathology. Many studies listed only the immunoreactivity of the nerve fibers, whereas others made deductions about nerve structure or type. In the latter, reports typically described thinly myelinated nerve endings, also known as Aδ-fibers (1/33) [17] or C-fibers (2/33) [17, 26] under the Erlanger-Gasser classification [54] within the annulus fibrosus. In other cases, nerves were described as myelinated or unmyelinated (6/33) [22, 26–28, 35, 47], encapsulated, partially encapsulated or unencapsulated (3/33) [17, 25, 30], by relative thickness and size (9/33) [17, 26, 29, 31, 32, 35–37, 40], or denoted by the Freeman classification (2/33) [31, 47, 55].
Perivascular fibers were reported in 10 studies, typically identified within the outer annulus fibrosus, and tended to be thinner than nerve fibers without adjacent blood vessels [27, 29, 32–34, 36, 38, 40, 44]. Blood vessels were identified by vascular endothelial growth factor (VEGF), CD31, CD34 immunoreactivity, or by counter-staining with hematoxylin. Of note, some studies did not report or specifically examine perivascular fibers so their presence may be significantly under-reported in the current scoping review.
Mechanoreceptors were highlighted in eight studies [27, 31, 33, 35, 46, 47, 50, 52] and were surmised to be of Golgi-type (proprioceptive), Ruffini-type (low-frequency vibration or pressure) or Pacini-type (high-frequency vibration or pressure) based on structural observations. These structures were found in the outer annulus fibrosus of both control and painful IVDs. Minimal data were presented regarding the precise location of these mechanoreceptors, and therefore no firm conclusion can be made regarding their topographical organization within the annulus fibrosus, or the relationship between these structures and the pathological state of the IVD.
Nucleus Pulposus
No neural elements were reported within the nucleus pulposus of control IVD tissues (23/33) across the lifespan, suggesting that it is a largely aneural structure in humans. In studies that did report neural elements within the nucleus pulposus (10/33) [17, 35, 36, 39–41, 49, 50, 52, 53], they were identified in degenerated IVDs and/or those from patients reporting back pain. One exception was Binch et al. who reported fibers positive for NF200 within the nucleus pulposus of both control and surgical tissues [49]. However, the health histories of the post-mortem control IVDs used in this study were not listed and therefore the presence of spine-related pathologies cannot be excluded [49]. The neural elements within the nucleus pulposus were described as free nerve endings [35, 50, 52], Ruffini corpuscles [50, 52], Aδ-fibers [17], C-fibers [17], or perivascular fibers [36, 40], and varied in their immunoreactivity depending on the specific report (detailed in Table 4).
Table 4.
Neural elements in the nucleus pulposus
| First Author | Year | Neural Markers and Stains | IVD Health Status | Description of Neural Elements |
|---|---|---|---|---|
| Roofe [22] | 1940 | Silver stain; gold chloride | … | … |
| Wiberg [23] | 1949 | Silver stain | … | … |
| Pedersen [24] | 1956 | Silver stain; Masson’s stain | … | … |
| Malinsky [25] | 1959 | Silver stain | … | … |
| Ferlic [26] | 1963 | Silver stain |
IVD degeneration … |
… |
| Jackson [27] | 1966 | Cholinesterase; silver stain |
IVD degeneration … |
… |
| Yoshizawa [28] | 1980 | Cholinesterase; silver stain |
LBP Similar problems (i.e., LBP) Normal IVD (control) |
… |
| Bogduk [29] | 1981 | Silver stain; osmium tetroxide | … | … |
| Bogduk [30] | 1988 | Silver stain; cholinesterase |
Spinal fusion surgery … |
… |
| Coppes [17] | 1990 | AChE; NF; SP |
IVD degeneration, LBP Spinal tumor surgery (control) |
|
| Mendel [31] | 1992 | Gold chloride | … | … |
| Ashton [32] | 1994 | PGP9.5; CGRP; SP; VIP; CPON | Back pain | … |
| Roberts [33] | 1995 | PGP9.5; CGRP; SP; VIP; NPY; Mayer’s hematoxylin |
LBP Scoliosis (control) … |
… |
| Brown [34] | 1997 | PGP9.5; CGRP; SP; NPY; H&E; toluidine blue |
IVD degeneration, LBP No chronic back pain (control) |
… |
| Coppes [35] | 1997 | AChE; NF90; SP |
IVD degeneration, LBP Spinal tumor surgery (control) |
|
| Freemont [36] | 1997 | PGP9.5; SP; GAP43; H&E |
Chronic back pain (split into pain and non-pain level) No spinal disorders (control) |
|
| Palmgren [37] | 1999 | SYN; PGP9.5; CPON; SP; hematoxylin | Non-degenerated IVDs | … |
| Johnson [38] | 2001 | PGP9.5; NF200; peripherin; GFAP; H&E |
Injury, IVD degeneration, LBP [all specimens allocated degeneration score] |
… |
| Bucknill [39] | 2002 | PGP9.5; NF; SNS/PN3; NaN/SNS2; neutral red | IVD degeneration or prolapse |
|
| Freemont [40] | 2002 | PGP9.5; p75; GAP43; Trk-A; toluidine blue |
IVD degeneration, pain IVD degeneration, no pain Normal IVD (control) |
|
| Peng [41] | 2005 | NF200; SP; VIP; hematoxylin |
LBP No LBP No spinal disorders (control) |
|
| Ohtori [42] | 2006 | PGP9.5 |
IVD degeneration or herniation, LBP Scoliosis, fracture |
… |
| Ozawa [43] | 2006 | PGP9.5; CGRP; IB4 | IVD degeneration or herniation, LBP | … |
| Johnson [44] | 2007 | NF200; pleiotrophin; Mayer’s hematoxylin |
IVD degeneration or prolapse, LBP Fracture, dislocation (control) [all specimens allocated degeneration score] |
… |
| Kokubo [45] | 2008 | NF68; GAP43; SP; NGF; H&E |
IVD herniation, pain Spondylosis, pain No spinal disorders (control) |
… |
| Dimitroulias [46] | 2010 | S100; Harris hematoxylin | No spinal disorders | … |
| Martins [47] | 2010 | S100; hematoxylin |
No spinal disorders [all specimens allocated degeneration score] |
… |
| Fields [48] | 2014 | PGP9.5; Heidenhain stain |
Endplate pathologies [all specimens allocated degeneration score] |
… |
| Binch [49] | 2015 | NF200; PGP9.5; H&E |
Nerve root compression Not listed (control) |
|
| Yang [50] | 2017 |
S100; NF200 Harris hematoxylin |
Cervical spondylosis with vertigo Cervical spondylosis without vertigo No spinal disorders (control) |
|
| Lama [51] | 2018 | PGP9.5; SP; H&E |
IVD herniation (sciatica) IVD herniation (no sciatica) IVD degeneration Scoliosis (control) [all specimens allocated degeneration score] |
… |
| Yang [52] | 2018 | S100; SP; H&E |
Transient neck pain and dizziness Acute spinal cord injury No spinal disorders (control) |
|
| Wu [53] | 2019 | SP; H&E |
Chronic neck pain Mild or no neck pain No spinal disorders (control) |
|
“…” represents insufficient information provided.
IVD=intervertebral disc; NP=nucleus pulposus; LBP=low back pain; H&E=hematoxylin and eosin; full names of neural marker acronyms, alongside functional descriptions, can be found in Table 2
Vertebral Endplate
Five studies included in the scoping review reported data on neural elements within the vertebral endplate [25, 34, 42, 48, 49]. From these, it was consistently reported that vertebral endplates of both control and pathological IVDs contained perivascular nerve fibers and nerve fibers not associated with blood vessels. Similar to the annulus fibrosus and nucleus pulposus, there was an increase in the number of nerve fibers detected in degenerated IVDs compared to controls. Fields et al. completed the most thorough investigation of the vertebral endplate, reporting that fibers positive for PGP9.5 were found in 82.6% (76/92) of the endplates examined and were consistently found with blood vessels that branched towards the endplate from the vertebral body [48]. Moreover, nerves were present in 90% (27/30) of endplate defects and 66.1% (35/53) of endplates with no defects or abnormal marrow elements [48]. The remaining reports corroborate these findings, demonstrating the presence of fibers positive for PGP9.5, NF200, SP or CGRP within the vertebral endplates of healthy and degenerate IVDs [34, 42, 49].
Temporal Changes in IVD Innervation
None of the studies included in the scoping review included age as a variable in the assessment of neural elements in the IVD. However, we cross-referenced findings of neural element localization from the included articles (data presented in Tables 3 and 4) with information provided on donor age to assess the possible association between IVD innervation and age. This analysis suggested that the IVD is modestly innervated until middle age, with neural elements predominantly localized in the outer one-third of the annulus fibrosus. In the absence of pathology, this innervation is detectable during the fetal period and increases into adolescence. In adults, age-related degenerative changes or spine-related pathologies likely lead to increased infiltration of nerve fibers into the inner annulus fibrosus and nucleus pulposus of the IVD which continue to proliferate with increasing degeneration or disease severity (Figure 2). However, since IVD degeneration results from the complex interplay between mechanical factors, genetic influences, and natural physiologic changes associated with aging [10, 56], it is not possible to associate increased innervation solely with age.
Discussion
Summary of Evidence
The objective of this scoping review was to summarize evidence related to the presence and organization of neural elements within the IVD across the lifespan. Overall, the IVD was found to be innervated across the lifespan, in both nonpathological and pathological tissues. Ingrowth of nerve fibers into the central tissues of the IVD was associated with painful (e.g., positive discography) and degenerate tissues, which were typically obtained from older patients. As such, changes in the presence of neural elements of the IVD with age and disease may have important implications for the pathogenesis of back pain and associated treatments.
Interpretation of Results
Relationship Between Nerve Location, Type, and the State of IVD Degeneration
The annulus fibrosus of most IVDs was innervated by a variety of different nerve fibers (Table 3) that appeared to increase in prevalence within tissues obtained from donors with a history of back pain. However, nerve infiltration into the inner annulus fibrosus and/or nucleus pulposus was almost entirely limited to painful or degenerate IVDs. Frequently, nerves were localized to areas of tissue granulation, annular tears, and lesions. This finding is consistent with the characterization of the blood vessels of the IVD [13] due to focal proteoglycan loss that is conducive to neurovascular ingrowth [57]. There did not appear to be a relationship between nerve type and location within control or degenerate IVDs, although this association was rarely directly examined and requires further investigation. Nevertheless, the diversity of nerve fiber types (as displayed by different neuropeptide markers) increased in degenerated or damaged IVDs, or those obtained from patients with back pain, compared to controls.
Association Between Neural Elements and Vascular Structures Within the IVD
The literature reviewed identified the presence of a large number of perivascular nerve fibers as well as nerve fibers not associated with blood vessels in the human IVD. Nerve fibers were not always associated with vasculature within the IVD. Both vasoregulatory and sensory nerve fibers were identified within the IVD, especially within IVD tissues from patients who reported back pain, where total innervation (i.e., all types of neural elements) was markedly higher than controls.
Methods Used to Study Neural Elements in the IVD
Neuropeptide antibodies such as SP and NPY are effective markers for neural elements within the IVD (see Table 2 for exhaustive list of antibodies used). However, it is critical that these markers are used in conjunction with general antibodies for neurons such as PGP9.5 and NF, and that all neural elements are co-labeled and presented with appropriate controls.
Identity of Neuronal Subtypes within the IVD
To identify neural structures, most articles employed general nerve markers that label common intracellular components specific to neurons or Schwann cells. These markers included PGP9.5, NF, PRPH, SYN, AChE, or S100 (Table 2). While these general markers did not provide insight into the precise identity of neural elements within the IVD, they facilitated the study of nerve thickness, and allowed the identification of different nerve endings based on their morphology. In addition to using general nerve markers, many studies also examined the localization of specific neuropeptides to assess the potential cell identity or state of growth, of neural elements within the IVD.
Substance P (SP) and Calcitonin Gene-Related Peptide (CGRP)
SP is a neuropeptide involved in primary afferent sensory neurotransmission [58] that is commonly reported to be expressed by nerves in IVDs obtained from patients who reported back pain [17, 32–37, 41, 45, 51–53]. A variety of neurons in the dorsal root ganglia synthesize and store SP in vesicles that are then delivered by fast axonal transport to peripheral nerve terminals [58]. In cases of inflammation or peripheral nerve injury, SP release upregulates NK1 receptors in dorsal horn neurons, which enhances peptidergic transmission and establishes hypersensitivity for the transmission of pain signals [59]. Given this well-established nociceptive role, it is likely that nerve fibers positive for SP in human IVDs are involved in the pain response. One report demonstrated increased gene expression of proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor (TNF)-α, matrix metalloproteinase (MMP)-10, nerve growth factor (NGF), and SP in the nucleus pulposus of degenerated IVDs from patients with low back pain, compared to controls (degenerated and nondegenerated post-mortem samples from patients with no documented history of back pain) [60]. The authors of this study suggested that cytokines such as IL-1 and TNF-α act synergistically to breakdown extracellular matrix via stimulation of MMP-10, which may consequently promote neoinnervation and nociception via NGF and SP, respectively [60]. CGRP is another neuropeptide localized to the IVD [32–34, 43], that contribute to processes of pain hypersensitivity and modulation [61]. Degenerated IVD tissues (categorized based on medical imaging and gross inspection) cultured ex vivo from patients with axial low back pain were shown to secrete increased levels of IL-1β, TNF-α, NGF, and brain-derived neurotrophic factor (BDNF) compared to IVD tissues from organ donors without back pain [62]. These factors increased neurite sprouting of PC12 cells and expression of CGRP in primary mouse DRG neurons [62]. Given that IVDs from patients with back pain contain nerve fibers positive for SP and/or CGRP, it is likely that these neural elements contribute to the onset or maintenance of discogenic pain.
Vasoactive Intestinal Peptide (VIP) and Neuropeptide-Y (NPY/CPON)
VIP is a neuropeptide widely expressed throughout the autonomic and enteric nervous systems. It is thought that VIP binds to VPAC1 or VPAC2 receptors on endothelial cells of capillaries, stimulating nitric oxide release and consequently vasodilation [63]. Like SP and CGRP, neural elements positive for VIP were found more frequently in IVDs from patients with back pain or IVD degeneration [32, 33, 41]. Previous research has shown that cytokine release (especially IL-1β) during IVD degeneration induces the expression of NGF and VEGF, a potent stimulator of angiogenesis [64]. NGF is known to promote neuronal proliferation, and VEGF expression may lead to ingrowth of endothelial cells which are known to express NGF, thus promoting innervation [64]. In this context, the presence of perivascular VIP-positive neurons within the degenerate IVD could be explained by degeneration-induced vascular ingrowth; these autonomic fibers may serve to innervate and regulate the nascent blood supply. In addition, NPY-positive perivascular nerve fibers were also localized within the outer annulus fibrosus of degenerate IVD specimens [33, 34]. In contrast to VIP, NPY is a strong vasoconstrictor, and is highly expressed in neurons of the sympathetic nervous system. NPY has also been shown to be angiogenic by acting through NPY2R receptors [65]. Similar to VIP, it is possible that NPY-positive neurons within the IVD [32–34, 37] proliferate in a neurotrophin-dependent manner, providing autonomic innervation to newly ingrown vasculature during the process of IVD degeneration.
Nerve Growth Factor (NGF) and Growth Associated Protein 43 (GAP-43)
While the present scoping review documents a variety of different neural elements within healthy and degenerate human IVDs, the mechanisms controlling neural ingrowth remain unclear. Freemont et al. demonstrated the presence of small perivascular fibers in the inner annulus fibrosus of painful IVDs (based on discography); these nerves expressed PGP9.5, GAP-43, and TrkA [40]. Kokubo et al. also reported similar nerve fibers positive for NGF or GAP-43 in the outer annulus fibrosus of IVD tissues from patients with radiculopathy, myelopathy or myeloradiculopathy [45]. The expression of nerve growth proteins such as NGF and GAP-43 in degenerated IVDs may implicate these mediators in processes of IVD innervation and nociception. In vitro studies demonstrate that co-culture of NP cells from painful and degenerated human IVDs with neuroblastoma cells increased the percentage of neurite-expressing cells and neurite length—effects that were dependent on NGF and BDNF, respectively [66]. Co-culture of neuroblastoma cells with annulus fibrosus cells from degenerated IVDs enhanced the secretion of VEGF, TGF-β1 and NGFβ compared to baseline culture conditions [67]. These findings led authors to propose that interactions between annulus fibrosus and neural cells enhance the production of growth factors responsible for neovascularization and nerve ingrowth during IVD degeneration [67]. Similarly, co-culture of F11 neural cells with annulus fibrosus cells from degenerate IVDs increased the expression of BDNF, neurotrophin receptors, and NGF receptors compared to levels from culture of F11 cells alone [68], providing further evidence to support the role of neurotrophins in mediating nerve-ingrowth into degenerate IVDs.
Gross Neuroanatomical Organization of Nerves Supplying the IVD
The variety of neuronal subtypes found within the IVD, and the functional implications related to the pain response, leads one to question their origins within the central nervous system. Two reports included in the scoping review reported gross dissection of nerves supplying IVD tissues [29, 30]. Four different sets of fibers were found to supply the lumbar IVDs. Branches from the ventral ramus supplied the posterolateral aspect of the IVD, while branches from the middle of the ramus communicans passed over the vertebral body to supply the lateral aspect of the IVD. Next, branches from the proximal end of the ramus communicans coursed in parallel with those from the middle and also supplied the lateral IVD. All of these fibers were distinct from branches of the sinuvertebral nerves, which also supplied the IVD [29, 30]. A subset of the reports also mentioned the neural supply to the paradiscal ligaments, structures which are known to be richly innervated [33]. These ligaments included the posterior longitudinal ligament [22–28, 30, 32, 41, 46], anterior longitudinal ligament [23, 25–29, 33, 35, 36, 38, 46, 50, 52], transverse ligament [30], and ligamentum flavum [23]. However, it remains unclear which specific nerves supply the paradiscal ligaments and which supply the IVD.
To our knowledge, no retrograde tracing studies have been conducted in humans; however, pre-clinical animal studies have investigated the neural pathways of fibers traveling from the spinal cord and adjacent paravertebral ganglia to the IVD. For example, in the rat L5/6 IVDs were injected with choleratoxin B and horseradish peroxidase; analysis of lumbar dorsal root ganglia revealed labeled neuronal cell bodies only within L1/2 ganglia, suggesting that sensory fibers innervating the IVD ascend via the sympathetic chain before re-entering sensory nerve roots at L1/2 [69]. Further research from this group demonstrated that nerve fibers from the L5/6 IVD in the rat traveled through both the sinuvertebral nerves and the sympathetic trunk to reach the dorsal root ganglia at L3-6 and T13/L2, respectively [70]. While further study is required in human tissues, the basic neuroanatomical findings described from blunt dissection studies in humans [29, 30] suggest possible sensory pathways for pain transmission.
Limitations in the Study of Neural Elements Within the IVD
Overall, a clear limitation in the study of neural elements within the IVD in the current scoping review was inconsistent annotation of data in the articles included for analysis. None of the studies stratified their findings by age or sex. Therefore, the potential influence of age and sex on the innervation of the IVD across the lifespan could not be accurately investigated. In addition, there was significant variability in the region of the IVD measured. Most reports only differentiated between the outer annulus fibrosus, inner annulus fibrosus, and nucleus pulposus, rather than documenting changes across the entire surface area of histological sections of the IVD. This is likely because many studies did not resect IVDs en bloc, but rather retrieved pieces of tissue that included a portion of the annulus fibrosus and nucleus pulposus, such as the anterior or lateral portion of the IVD. This limitation is inherent to the use of surgically-resected, degenerate IVDs from patients who experienced back pain.
Some studies did not report on the localization of nerve fiber types (immunoreactivity) within the annulus fibrosus, nucleus pulposus, or vertebral endplate. In addition, many studies used general nerve markers (i.e., PGP9.5) and specific nerve markers (i.e., SP) to investigate the presence of neural elements; however, these markers were not co-localized. It is possible that some markers such as SP may not have labelled neurons, as eosinophils, macrophages, T cells, and dendritic cells have all been shown to express this neuropeptide [71]. In studies that did use a co-labelling approach [36, 40, 41, 50], the percentage of co-labeled cells was not indicated.
In some cases, studies reported nerves as being myelinated or non-myelinated without using reliable stains (osmium tetroxide; luxol fast blue), antibodies (myelin oligodendrocyte glycoprotein; myelin basic protein) or appropriate microstructural imaging techniques such as electron microscopy [27, 35, 38, 47, 48]. For this reason, the myelination of nerve fibers within the IVD requires further study to confirm the reported observations. Finally, while some reports used reliable histological or MRI-based grading systems to assess the level of degeneration of the IVD tissues [38, 40, 44, 47, 48, 51], many reports segregated IVDs into controls and painful groups based exclusively on the available health history. This may have led to heterogeneity in the degeneration levels of tissues that were included in the distinct groups, since clinical studies establish a high incidence of asymptomatic IVD degeneration [72].
Limitations of the Current Scoping Review
The current scoping review was limited in its ability to interpret non-English articles that may have included pertinent information regarding neural elements within the IVD (25 articles total; see Figure 1). Further, the search strategy was not designed to identify studies detailing vascular structures in the IVD, although these studies were not excluded if nerves were also described. It is therefore possible that the search did not return comprehensive results for perivascular nerves or any neural elements associated with blood vessels within the IVD. Further, the scoping review intended to document only the neural elements reported in human tissues and therefore does not include molecular or mechanistic insights from animal studies or in vitro analyses.
Recommendations for Future Research
It is critical that future research undertake the study of neural elements within the IVD to discern the precise roles of neurons found in healthy and degenerated IVDs. Immunohistochemical techniques should include co-labeling of general and specific neuronal markers to ensure accurate identification of neural elements, and entire IVDs should be analyzed en bloc to assess neuronal topography in both 2D and 3D (although it is recognized that this will limit the study of IVDs to cadaveric or fresh autopsy materials). Additional markers for vasculature, circulating immune cells, and connective tissue cells can be used to enhance understanding of the microenvironment in which neural elements exist within the degenerate human IVD. Further, IVDs should be grouped according to age, sex, and degree of degeneration in order to investigate the influence of these variables on neural in-growth. Such studies could be complimented by microstructural analyses using electron microscopy, multiphoton fluorescence microscopy, light sheet microscopy [73, 74], or contrast-enhanced imaging that employs a variety of modern techniques (e.g., micro-CT and high-field MRI); these methods would allow for more accurate descriptions of neural cell identity and precise 3D reconstructions of neural networks within the IVD.
Conclusion
It is clear that the human IVD is an innervated structure throughout life and that neural ingrowth is associated with the state of tissue degeneration or damage. Neural elements were associated with blood vessels but also coursed in isolation within the IVD tissue. The most effective method to study neural elements within the IVD was histology and immunohistochemistry. The association between neural ingrowth and variables such as sex and age requires further study; in addition, precise characterizations of neuronal subtypes (such as those described in the current review) within the human IVD will allow for insight into their potential role in nociceptive signaling. Overall, the local changes to the IVD that occur with ageing, degeneration, and damage are associated with increased innervation of the IVD and may lead to discogenic back pain.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Western Libraries and Inter-library Loan services for assistance retrieving articles.
Supplementary Data
Supplementary data are available at Pain Medicine online.
Funding source: AMRG was supported by a Richard H. Tomlinson Doctoral Fellowship from the Faculty of Medicine and Health Sciences at McGill University. DEF was supported in part by a Transdisciplinary Training Award from the Bone and Joint Institute at The University of Western Ontario, and a Ph.D. Award from the Arthritis Society (No. 19–0469). MCB is the Western Research Chair in Exercise, Mobility and Musculoskeletal Health affiliated with Western’s Bone and Joint Institute. CAS is supported by a Career Development Award from the Arthritis Society and Early Researcher Award from the Ontario Ministry of Research and Innovation. This study was supported by the Canadian Institutes of Health Research (No. 115068).
Conflict of interest: None.
References
- 1. Herkowitz HN, Dvorak J, Bell GR, Nordin M, Grob D.. Epidemiology and the economics of low back pain. In: Epidemiology and the Economics. Philadelphia: Lippincott Williams & Wilkins; 2004:3–10. [Google Scholar]
- 2. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou R, Shekelle P.. Will this patient develop persistent disabling low back pain? JAMA 2010;303(13):1295–302. [DOI] [PubMed] [Google Scholar]
- 4. Deyo RA, Weinstein JN.. Low back pain. N Engl J Med 2001;344(5):363–70. [DOI] [PubMed] [Google Scholar]
- 5. Deyo RA, Mirza SK, Martin BI.. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31(23):2724–7. [DOI] [PubMed] [Google Scholar]
- 6. Qaseem A, Wilt TJ, McLean RM, Forciea MA, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American college of physicians. Ann Intern Med 2017;166(7):514–30. [DOI] [PubMed] [Google Scholar]
- 7. Fujii K, Yamazaki M, Kang JD, et al. Discogenic back pain: Literature review of definition, diagnosis, and treatment. JBMR Plus 2019;3(5):e10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Prim 2018;4(1):1–18. [DOI] [PubMed] [Google Scholar]
- 9. Risbud MV, Shapiro IM.. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adams MA, Roughley PJ.. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31(18):2151–61. [DOI] [PubMed] [Google Scholar]
- 11. Urban JP. Present perspectives on cartilage and chondrocyte mechanobiology. Biorheology 2000;37(1–2):185–90. [PubMed] [Google Scholar]
- 12. Urban JPG, Smith S, Fairbank JCT.. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29(23):2700–9. [DOI] [PubMed] [Google Scholar]
- 13. Fournier DE, Kiser PK, Shoemaker JK, Battié MC, Séguin CA.. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020;3(4):e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J Anat 2010;217(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohtori S, Miyagi M, Inoue G.. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg Relat Res 2018;2(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 17. Coppes MH, Marani E, Thomeer RTWM, Oudega M, Groen GJ.. Innervation of annulus fibrosis in low back pain. Lancet 1990;336(8708):189–90. [DOI] [PubMed] [Google Scholar]
- 18. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK.. Intervertebral disk degeneration and repair. Neurosurgery 2017;80(3S):S46–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H.. Chapter 11: Scoping reviews. In: JBI Manual for Evidence Synthesis. JBI 2020. doi: 10.46658/JBIMES-20-12. [Google Scholar]
- 20. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20(1):37–46. [Google Scholar]
- 21. Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159. [PubMed] [Google Scholar]
- 22. Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament: Fourth and fifth lumbar level. Arch Neurol Psychiatry 1940;44(1):100–3. [Google Scholar]
- 23. Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand 1949;19(2):211–21. [DOI] [PubMed] [Google Scholar]
- 24. Pedersen HE, Blunck CFJ, Gardner E.. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerve (sinu-vertebral nerves); with an experimental study of their functions. J Bone Jt Surg 1956;38 (2):377–91. [PubMed] [Google Scholar]
- 25. Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat (Basel) 1959;38: 96–113. [PubMed] [Google Scholar]
- 26. Ferlic D. The nerve supply of the cervical intervertebral disc in man. Bull Johns Hopkins Hosp 1963;113:347–51. [PubMed] [Google Scholar]
- 27. Jackson HC, Winkelmann RK, Bickel W.. Nerve endings in the human lumbar spinal column and related structures. J Bone Jt Surg 1966;48A(7):1272–81. [PubMed] [Google Scholar]
- 28. Yoshizawa H, O'Brien JP, Thomas Smith W, Trumper M.. The neuropathology of intervertebral discs removed for low-back pain. J Pathol 1980;132(2):95–104. [DOI] [PubMed] [Google Scholar]
- 29. Bogduk N, Tynan W, Wilson AS.. The nerve supply to the human lumbar intervertebral discs. J Anat 1981;132(Pt 1):39–56. [PMC free article] [PubMed] [Google Scholar]
- 30. Bogduk N, Windsor M, Inglis A.. The innervation of the cervical intervertebral discs. Spine (Phila Pa 1976) 1988;13(1):2–8. [DOI] [PubMed] [Google Scholar]
- 31. Mendel T, Wink CS, Zimny ML.. Neural elements in human cervical intervertebral discs. Spine (Phila Pa 1976) 1992;17(2):132–5. [DOI] [PubMed] [Google Scholar]
- 32. Ashton IK, Roberts S, Jaffray DC, Polak JM, Eisenstein SM.. Neuropeptides in the human intervertebral disc. J Orthop Res 1994;12(2):186–92. [DOI] [PubMed] [Google Scholar]
- 33. Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK.. Mechanoreceptors in intervertebral discs: Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976) 1995;20(24):2645–51. [DOI] [PubMed] [Google Scholar]
- 34. Brown MF, Hukkanen MVJ, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Jt Surg - Ser B 1997;79-B(1):147–53. [DOI] [PubMed] [Google Scholar]
- 35. Coppes MH, Marani E, Thomeer RT, Groen GJ.. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976) 1997;22(20):2342–50. [DOI] [PubMed] [Google Scholar]
- 36. Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MIV.. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997;350(9072):178–81. [DOI] [PubMed] [Google Scholar]
- 37. Palmgren T, Gronblad M, Virri J.. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976) 1999;24(20):2075–9. [DOI] [PubMed] [Google Scholar]
- 38. Johnson W, Evans H, Menage J, Eisenstein S, El Haj A, Roberts S.. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26(23):2550–7. [DOI] [PubMed] [Google Scholar]
- 39. Bucknill AT, Coward K, Plumpton C, et al. Nerve fibers in lumbar spine structures and injured spinal roots express the sensory neuron-specific sodium channels SNS/PN3 and NaN/SNS2. Spine (Phila Pa 1976) 2002;27(2):135–40. [DOI] [PubMed] [Google Scholar]
- 40. Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol 2002;197(3):286–92. [DOI] [PubMed] [Google Scholar]
- 41. Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y.. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br 2005;87-B(1):62–7. [PubMed] [Google Scholar]
- 42. Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and modic type 1 or type 2 changes on MRI. Spine (Phila Pa 1976) 2006;31(9):1026–31. [DOI] [PubMed] [Google Scholar]
- 43. Ozawa T, Ohtori S, Inoue G, Aoki Y, Moriya H, Takahashi K.. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976) 2006;31(21):2418–22. [DOI] [PubMed] [Google Scholar]
- 44. Johnson WEB, Patterson AM, Eisenstein SM, Roberts S.. The presence of pleiotrophin in the human intervertebral disc is associated with increased vascularization: An immunohistologic study. Spine (Phila Pa 1976) 2007;32(12):1295–302. [DOI] [PubMed] [Google Scholar]
- 45. Kokubo Y, Uchida K, Kobayashi S, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: Histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine 2008;9(3):285–95. [DOI] [PubMed] [Google Scholar]
- 46. Dimitroulias A, Tsonidis C, Natsis K, et al. An immunohistochemical study of mechanoreceptors in lumbar spine intervertebral discs. J Clin Neurosci 2010;17(6):742–5. [DOI] [PubMed] [Google Scholar]
- 47. Martins DE, de Oliveira VM, de Alves MS, et al. Correlations between radiographic, magnetic resonance and histological examinations on the degeneration of human lumbar intervertebral discs. Sao Paulo Med J 2010;128(2):63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fields A, Liebenberg E, Aaron J, Liebenberg E, Lotz J.. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J 2014;14(3):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Binch A, Cole A, Breakwell L, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther 2015;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang L, Yang C, Pang X, et al. Mechanoreceptors in diseased cervical intervertebral disc and vertigo. Spine (Phila Pa 1976) 2017;42(8):540–6. [DOI] [PubMed] [Google Scholar]
- 51. Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA.. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat 2018;233(1):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang L, Chen J, Yang C, et al. Cervical intervertebral disc degeneration contributes to dizziness: A clinical and immunohistochemical study. World Neurosurg 2018;119:e686–93. [DOI] [PubMed] [Google Scholar]
- 53. Wu B, Yang L, Peng B.. Ingrowth of nociceptive receptors into diseased cervical intervertebral disc is associated with discogenic neck pain. Pain Med 2019;20(6):1072–7. [DOI] [PubMed] [Google Scholar]
- 54. Kandel ER, Schwartz JH, Jessell TM.. Principles of Neural Science. Vol. 4. 2nd edition. New York: McGraw-Hill; 2000. [Google Scholar]
- 55. Freeman MAR, Wyke B.. The innervation of the ankle joint. An anatomical and histological study in the cat. Cells Tissues Organs 1967;68(3):321–33. [DOI] [PubMed] [Google Scholar]
- 56. Battié MC, Videman T, Parent E.. Lumbar disc degeneration: Epidemiology and genetic influences. Spine (Phila Pa 1976) 2004;29(23):2679–90. [DOI] [PubMed] [Google Scholar]
- 57. Stefanakis M, Al-Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976) 2012;37(22):1883–91. [DOI] [PubMed] [Google Scholar]
- 58. Zieglgänsberger W, Substance P, Pain C.. Substance P and pain chronicity. Cell Tissue Res 2019;375(1):227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weisshaar CL, Winkelstein BA.. Ablating spinal NK1-bearing neurons eliminates the development of pain and reduces spinal neuronal hyperexcitability and inflammation from mechanical joint injury in the rat. J Pain 2014;15(4):378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richardson SM, Doyle P, Minogue BM, et al. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther 2009;11(4):R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen LJ, Zhang FG, Li J, et al. Expression of calcitonin gene-related peptide in anterior and posterior horns of the spinal cord after brachial plexus injury. J Clin Neurosci 2010;17(1):87–91. [DOI] [PubMed] [Google Scholar]
- 62. Krock E, Rosenzweig D, Chabot-Dore A, et al. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med 2014;18(6):1213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iwasaki M, Akiba Y, Kaunitz JD.. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000 Res 2019;8:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Binch ALA, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther 2014;16(4):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 2007;13(7):803–11. [DOI] [PubMed] [Google Scholar]
- 66. Richardson SM, Purmessur D, Baird P, Probyn B, Freemont AJ, Hoyland JA.. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PLoS One 2012;7(10):e47735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moon HJ, Kim JH, Lee HS, et al. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine (Phila Pa 1976) 2012;37(1):2–9. [DOI] [PubMed] [Google Scholar]
- 68. Gruber H, Hoelscher G, Bullock L, Ingram J, Norton H, Hanley E.. Human annulus signaling cues for nerve outgrowth: In vitro studies. J Orthop Res 2016;34(8):1456–65. [DOI] [PubMed] [Google Scholar]
- 69. Morinaga T, Takahashi K, Yamagata M, et al. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine (Phila Pa 1976) 1996;21(16):1848–51. [DOI] [PubMed] [Google Scholar]
- 70. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H.. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine (Phila Pa 1976) 1999;24(22):2295–9. [DOI] [PubMed] [Google Scholar]
- 71. Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R.. Neuropeptide substance P and the immune response. Cell Mol Life Sci 2016;73(22):4249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS.. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331(2):69–73. [DOI] [PubMed] [Google Scholar]
- 73. Huisken J, Swoger J, Del BF, Wittbrodt J, Stelzer EHK.. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science (80-) 2004;305(5686):1007–9. [DOI] [PubMed] [Google Scholar]
- 74. Fadero TC, Gerbich TM, Rana K, et al. LITE microscopy: Tilted light-sheet excitation of model organisms offers high resolution and low photobleaching. J Cell Biol 2018;217(5):1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.