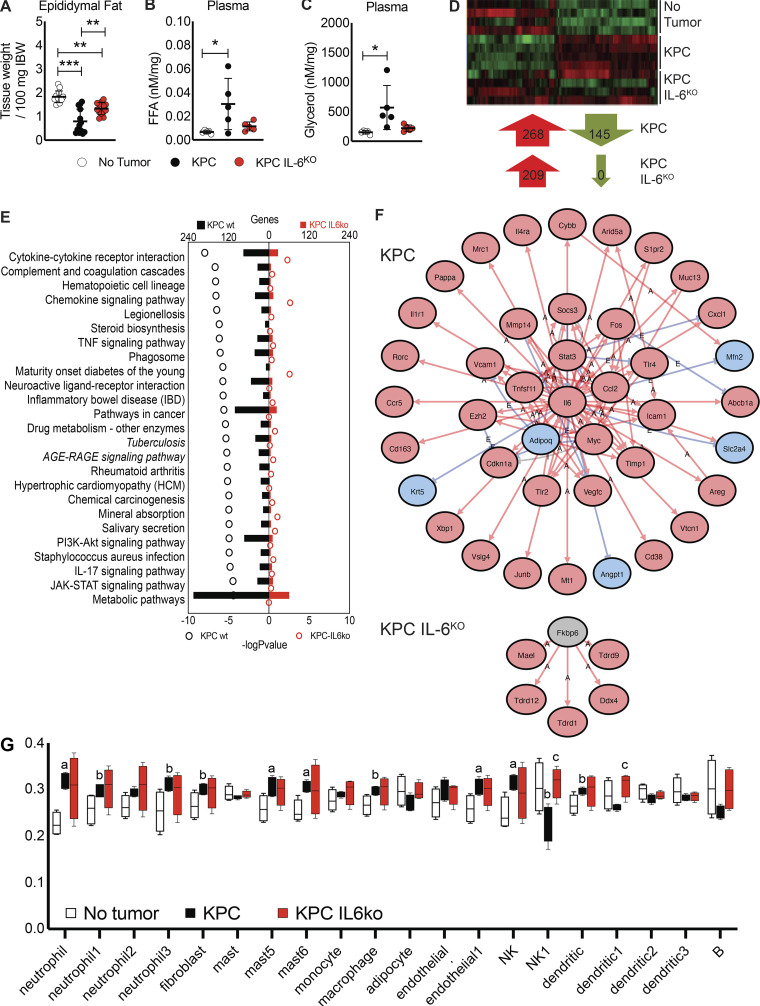
Figure 6.
Deletion of IL-6 from KPC cells reduces fat wasting and alters affected molecular pathways and upstream regulators in adipose tissue. (A) Epididymal fat pad mass was measured at euthanasia and normalized to initial body weight (IBW). Data represent the mean and SD from two separate experiments (n = 13 per group), and statistical differences (**, P < 0.01; ***, P < 0.0001) were determined using ANOVA and Tukey’s multiple comparisons test. FFA, free fatty acid. (B and C) Characterization of lipolysis using measurements of plasma glycerol (B) and FAs (C) normalized to epididymal fat pad mass from mice. Data represent mean and SD from a single experiment (n = 5 per group), and statistical differences (*, P < 0.05) were determined using ANOVA and Tukey’s multiple comparisons test. (D) Isolated RNA from the epididymal fat pads (n = 4 per group) was sequenced, and differentially regulated genes (compared with no tumor group, FC ≥ |1.5| and FDR ≤ 0.05) were compared across groups. (E) iPathway analysis using epididymal fat pad RNA-seq data identified differentially regulated pathways. (F) iPathway also identified upstream regulators in the adipose tissue of KPC and KPC IL6KO tumor mice. Red arrows labeled with A indicate an activating interaction, while blue arrows labeled with E indicate an inhibitory interaction. (G) Deconvolution analysis of the RNA-seq data identified changes in cell subtypes present in the adipose tissue; mast, mast cells; B, B cells; a, P < 0.05 versus no tumor; b, P < 0.10 versus no tumor; and c, P < 0.05 versus KPC.