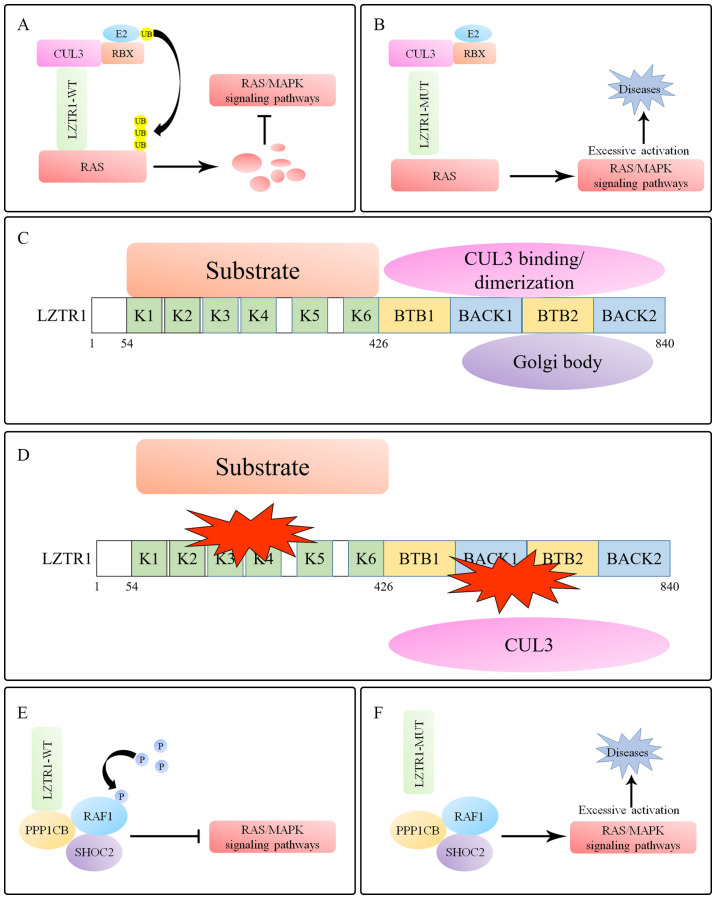
Figure 1.
LZTR1 regulates RAS/MAPK signaling. (A) LZTR1 induces polyubiquitination and degradation of RAS proteins to inhibit the RAS/MAPK signaling pathway. (B) Mutated LZTR1 loses the ability to regulate RAS superfamily proteins, leading to excessive activation of the RAS/MAPK signaling pathway. (C) A total of six N-terminal Kelch motifs and two BACK domains are located at the C-terminus within LZTR1. The Kelch domains selectively recruit substrates, whereas the BACK domains are predicted to mediate dimerization and CUL3 binding. (D) Mutations in Kelch domains decrease binding to substrates. The mutations located in the BTB/BACK domains of LZTR1 prevent the binding of LZTR1 to CUL3. All of these mutations prevent the formation of the substrate-LZTR1-CUL3 complex. (E) LZTR1 mediates RAF phosphorylation by binding to the RAF1/PPP1CB/SHOC2 complex to inhibit the RAS/MAPK signaling pathway. (F) Mutations in LZTR1 lead to loss of RAF1 regulation, leading to excessive activation of the RAS/MAPK signaling pathway, eventually resulting in Noonan syndrome. LZTR1, leucine zipper-like transcription regulator 1; BTB, broad-complex, tramtrack, and bric-a-brac; BACK, BTB and C-terminal Kelch; CUL, cullin; PPP1CB, protein phosphatase 1 catalytic subunit β; RBX, RING box protein; WT wild-type; Mut, mutant; UB, ubiquitin; P, pyrophosphate.