Abstract
BACKGROUND AND PURPOSE: Viable tumor in a neck dissection specimen is important in predicting prognosis and directing treatment. Our purpose was to clarify the importance of size changes of regional metastases from head and neck squamous cell carcinoma on CT scans obtained before and after radiation therapy (RT) as a predictor of pathologic outcome.
METHODS: Thirty-seven heminecks in 34 patients who underwent pre-RT CT, RT, post-RT CT, and post-RT neck dissection were reviewed. Thirteen hemineck specimens were pathologically positive. Decrease ratios of the largest axial dimension of the lymph nodes between the pre- and post-RT CT studies were calculated.
RESULTS: Six of 37 heminecks had a decrease ratio greater than 50%. These yielded negative specimens after planned neck dissection. In two of 37 heminecks, the largest axial dimension of the largest node increased between studies, resulting in negative decrease ratio. One (decrease ratio, −20%) had a positive specimen, and the other (decrease ratio, −3%) had a negative specimen. No interval change in size in the largest node was noted in one of the 37 heminecks; its specimen was positive. Average decrease ratios were 41.2% (range, −3% to 62%) in the negative specimen group (n = 24) and 27.2% (range, −20% to 50%) in the positive specimen group (n = 13). Univariate analysis revealed that the decrease ratio was not a significant predictor of a positive surgical specimen (P = .154).
CONCLUSION: Heminecks in which the decrease ratio was greater than 50% tended to have a negative surgical specimen. However, this trend was not statistically significant.
Nodal metastasis in squamous cell carcinoma of the head and neck is one of the most challenging management issues for both head-and-neck oncologists and radiologists. Definitive diagnosis, even with modern imaging modalities, remains an unfulfilled goal. The standard treatment strategy for each clinical setting varies depending on the patient’s desires, the primary site and extent of the tumor, the extent of nodal disease, and the institutional and/or practitioner biases.
High-dose radiation therapy (RT) followed by neck dissection has emerged as a generally accepted option, especially in patients with relatively advanced neck disease (1). With this approach has come controversy with regard to the necessity of planned post-RT neck dissection, especially when the original N stage is relatively low and/or if the clinical response is complete.
Patients with a negative surgical specimen after RT are more likely to have ultimate control of neck disease than those with a positive specimen (1). The objective of this study was to clarify whether a percentage decrease ratio related to the largest axial dimension of the largest lymph node in each hemineck can be used to predict the likelihood of residual, pathologically identifiable, neck disease in patients with head and neck squamous cell carcinoma. Such information might help in determining the necessity of planned post-RT neck dissection.
Methods
We reviewed hospital records of 232 patients who underwent either radical or modified neck dissection within 2 months after high-dose RT for squamous cell carcinoma of the head and neck region between January 1, 1990, and December 31, 1999. This study included patients with squamous cell carcinoma at head and neck sites (excluding the nasopharynx, skin, and sinonasal region).
Patients with synchronous or metachronous secondary primary tumors were excluded. All patients in this study had undergone pre-RT CT, RT with curative intent, post-RT CT, and post-RT neck dissection within 2 months after RT. Procedures were performed at the University of Florida, in this order. Analyses were performed with a hemineck as a unit.
Thirty-seven heminecks in 34 of these 232 patients were eligible for analysis. The study population consisted of 28 male and six female patients with an average age of 58 years (range, 28–87 years). The main reasons for excluding the other 198 patients were a lack of necessary clinical data in their hospital charts, nonqualifying primary sites, and lack of appropriate CT studies.
Thirty-two of the 34 patients had undergone twice-a-day continuous RT, and the other two had undergone once-a-day continuous RT. No patient had a split treatment course. The average overall treatment time was 46.3 days (range, 33–54 days), and the total dose of RT was 7168.2 cGy (range, 5580–7920 cGy).
Primary sites in the 34 patients were the oral cavity (two patients), the oropharynx (20 patients), the hypopharynx (three patients), the larynx (three patients), and unknown primary (six patients).
Tumor differentiation was based on histologic examination of pre-RT biopsy samples in the 34 patients. Results indicated well or well to moderately differentiated carcinoma (two patients), moderately differentiated carcinoma (11 patients), and poorly or moderately to poorly differentiated carcinoma (11 patients). No definitive pathologic statement was available with regard to the differentiation in the other 10 patients.
Two blinded head and neck radiologists (H.O., A.A.M.) retrospectively reviewed all CT images with the data collected by reaching a consensus on all findings and measurements. The average interval between the completion of RT and post-RT CT was 30.9 days (range, 13–49 days). In all patients, CT images were obtained after the intravenous administration of iodinated contrast material. CT images were typically obtained with 3-mm thickness at 5-mm increments to evaluate the neck outside the primary site and with contiguous 3-mm sections to evaluate the primary site and encompassed nodal levels.
Only heminecks with a cervical lymph node or nodes that was 20 mm or larger at any level on pre-RT CT scans and that would undergo post-RT neck dissection were entered in the database. This criterion was used to minimize the possibility of including false-positive cases in the analysis. The largest axial dimension in the largest node was measured on axial CT images. In total, 37 heminecks in 34 patients had surgical specimens after high-dose RT that were available for review. Primary sites and the T and N stages of the heminecks are summarized in Table 1. Six heminecks (in five patients) were also treated with preoperative chemotherapy. Three heminecks (in two patients) were treated with induction chemotherapy, and three heminecks (in three patients) were treated with concomitant chemotherapy.
TABLE 1:
Number of heminecks in each primary site, and T- and N-stages
| Characteristic | No. of Heminecks (N = 37) |
|---|---|
| Primary site | |
| Oral cavity | 2 |
| Oropharynx | 22 |
| Hypopharynx | 3 |
| Larynx | 4 |
| Unknown | 6 |
| T stage | |
| T0 | 6 |
| T1 | 8 |
| T2 | 13 |
| T3 | 3 |
| T4 | 7 |
| N stage | |
| N0 | 0 |
| N1 | 1 |
| N2A | 8 |
| N2B | 19 |
| N3 | 9 |
Thirteen heminecks (in 13 of 35 patients) were histologically positive for residual metastatic squamous cell carcinoma. In all 13 positive heminecks, the neck level that was positive in surgical specimens included the region containing the largest lymph node on the pre-RT CT image
On clinical assessment, the post-RT status was a complete response in seven heminecks, a partial response in 30 heminecks, no change in 0 patients, and progressive disease in 0 patients. The response to RT was clinically evaluated at 6 weeks after the completion of high-dose RT.
We calculated the percentage decreased ratio by comparing the largest axial dimension of the largest lymph node in each hemineck on the pre-RT CT scans with the largest axial dimension of what was presumably the same node on post-RT CT scans (Fig 1). Our purpose was to correlate the ratio with the pathologic outcome of planned post-RT neck dissection.
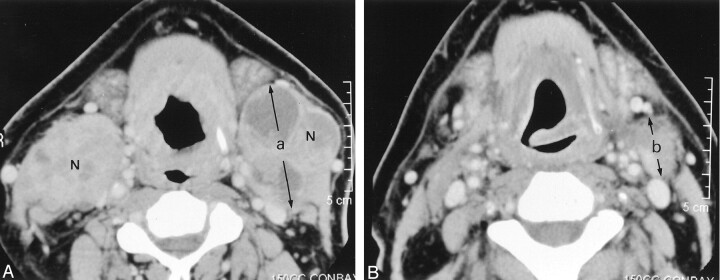
Fig 1.
Images in a 46-year-old man with a carcinoma at the base of his tongue (T4N3[3, 3]). (The percentage decrease ratio was calculated as in follows: % decrease ratio = (1 - b/a) × 100, where a and b are the dimensions shown in images A and B, respectively.
A, Pre-RT contrast-enhanced CT image reveals enlarged level 2 lymph nodes (N) on both sides. The one on the right measures 54 × 33 mm, and that on the left, 57 × 45 mm. The nodes contain focal areas of low attenuation. An infiltrative mass involves the lower part of the base of the tongue.
B, Post-RT contrast-enhanced CT image shows a marked decrease in the size of the lymph nodes. The decrease ratio of the largest dimension of the nodes is 59% on the right and 51% on the left. The patient underwent planned post-RT neck dissection on both sides. The surgical specimens were negative on both sides.
Statistical Analysis
Univariate analysis was performed to determine the relative role of the variable, that is, the percentage decrease ratio in the largest dimension of the largest node in each hemineck from pre- to post-RT CT scans, in the pathologic outcome of planned post-RT neck dissection. ÷2 test statistics were used.
Results
The distribution of the largest axial dimension of the largest node in each hemineck on both pre- and post-RT CT images is summarized in Table 2.
TABLE 2:
Largest axial dimension of the largest node in the 37 heminecks on pre- and post-RT CT scans
| Largest Dimension, mm | No. of Heminecks |
|
|---|---|---|
| On Pre-RT CT Scans | On Post-RT CT Scans | |
| <19 | 0 | 14 |
| 20–29 | 14 | 18 |
| 30–39 | 10 | 2 |
| 40–49 | 7 | 3 |
| >50 | 6 | 0 |
The pathologic outcome of planned post-RT neck dissection and the percentage decrease ratio in the largest dimension of the largest node in each hemineck from pre- to post-RT CT image are as shown in Table 3. None of six heminecks in which the decrease ratio was more than 50% had a positive surgical specimen (Figs 1 and 2). The average decrease ratio was 41.2% (range, −3% to 62%) in the negative specimen group (n = 24) and 27.2% (range, −20% to 50%) in the positive specimen group (n = 13) (Fig 3). The decrease ratio was 0% in one hemineck and negative in two heminecks. The calculated negative percentage decrease ratio indicated an interval growth in a node after RT, and the 0% decrease ratio indicated no interval change in nodal size. Two of the three heminecks (decrease ratios, 0% and −20%) had a positive surgical specimen (Fig 4). The other (decrease ratio, −3%; size change, 40 to 41 mm) had a negative specimen. All three were classified as having a partial response on clinical assessment. Most of nodes regressed to some degree, but the largest one showed no obvious interval change in two heminecks (decrease ratios, 0% and −3%). Most of the measured nodes regressed after RT, but only the largest one increased in its largest axial dimension from the pre-RT CT study to the post-RT CT study (decrease ratio, −20%) (Fig 4).
TABLE 3:
Pathologic outcome and percentage decrease ratio in the largest axial dimension of the largest node in each hemineck
| Decrease Ratio, % | No. of Negative Specimens (n = 24) | No. of Positive Specimens (n = 13) | Total (N = 37) |
|---|---|---|---|
| <30 | 4 | 6 | 10 |
| 31–40 | 6 | 3 | 9 |
| 41–50 | 8 | 4 | 12 |
| >50 | 6 | 0 | 6 |
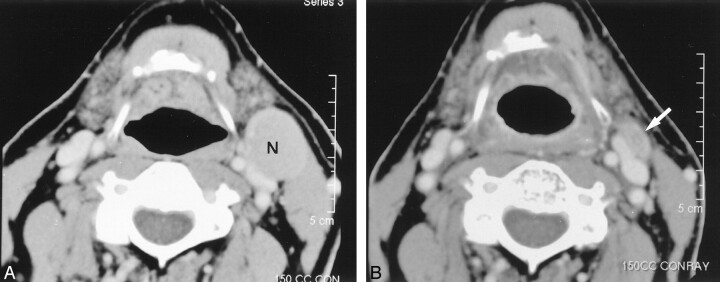
Fig 2.
Images in a 64-year-old man with metastatic neck disease from an unknown primary squamous cell carcinoma (TXN2A).
A, Pre-RT contrast-enhanced CT image reveals an enlarged (27 × 22 mm) level 2 lymph node (N) on the left.
B, Post-RT contrast-enhanced CT image shows a small residual mass (arrow) (decrease ratio of the largest dimension of the node, 52%). The patient underwent left neck dissection after this study. The specimen from the left hemineck was negative.
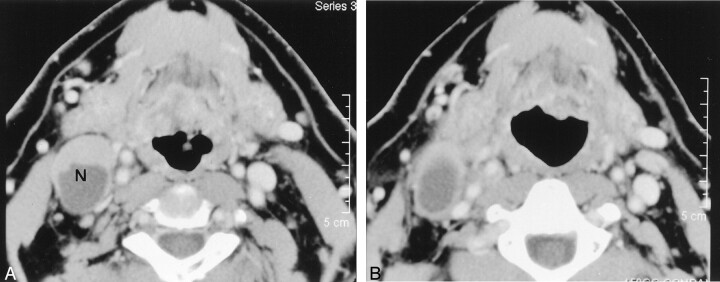
Fig 3.
Images in a 52-year-old man with tongue base carcinoma (T2N2B).
A, Pre-RT contrast-enhanced CT image shows the enlarged (34 × 24 mm) level 2 lymph node (N) on the right. The node contains a focal area of low attenuation.
B, Post-RT contrast-enhanced CT image reveals a minimal decrease in the size of the lymph node (decrease ratio of the largest dimension of the node, 6%). The surgical specimen from planned post-RT neck dissection at level 2 of the right hemineck was positive.
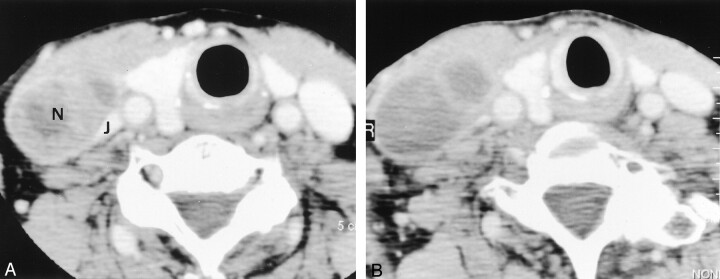
Fig 4.
Images in a 78-year-old woman with pyriform sinus carcinoma (T2N2A).
A, Pre-RT contrast enhanced CT image shows an enlarged (40 × 25 mm) level 4 lymph node (N) on the right. The nodal mass distorts the adjacent right internal jugular vein (J).
B, Post-RT contrast-enhanced CT image shows a slight increase in the size of the lymph node (decrease ratio of the largest dimension, −20%). The internal attenuation is generally lower on this image than on the image in A. The specimen from the right hemineck was positive.
Univariate analysis revealed that the decrease ratio is not a significant predictor of positive surgical specimens from planned post-RT neck dissection (P = .154).
Discussion
For certain primary sites and tumor stages in the head and neck, definitive RT with or without chemotherapy is the treatment of choice (1). In this setting, management of an initially positive neck is somewhat controversial, with tradition dictating a planned neck dissection for an initially positive neck size. However, current imaging techniques, which can be used to monitor changes in nodal size after RT, may indicate a certain level of response to RT; if present, this result may obviate planned neck dissection.
Patients with negative surgical specimens from the planned post-RT neck dissection are more likely to have ultimate control of neck disease than those with a positive specimen (2). Because surgery succeeds in less than 10% of patients with initially positive specimens in whom RT fails (3), the pathologic outcome of the planned post-RT neck dissection specimen is an important predictor of the prognosis and the treatment strategy in this group.
Mendenhall et al (2) reported that the percentage of positive specimens was not correlated with the size of the largest nodal mass, although they noted a general trend for more positive specimens with increased size. This observation was also true in another report by Boyd et al (4). In addition, Mendenhall et al (2) reported that node size was not related to failure in the negative specimen group.
None of six heminecks in which a the decrease ratio was greater than 50% had a positive surgical specimen from the planned post-RT neck dissection (Figs 1 and 2). Although the current findings suggest that heminecks with a decrease ratio of greater than 50% have a tendency to have a negative surgical specimen, the ratio is not a statistically significant predictor of surgical specimen status, as determined at univariate analysis (P = .154).
Mendenhall et al (2) suggested that the rapid and complete response of the nodal metastasis to RT might be a predictor of ultimate control by means of RT alone.
Labadie et al (5) reported that the volume reduction in metastatic nodes (91% ± 4%) in patients with squamous cell head and neck cancer who underwent concurrent chemotherapy and RT was significantly greater than that of normal nodes (55% ± 21%) (P > .02, two-sided t test). They also suggested that significant nodal volume reduction correlated with a high degree of negative pathologic findings; only one of 19 previously clinically positive lymph nodes has a positive surgical specimen. Their study population (n = 7) was specifically defined as patients who underwent concurrent chemotherapy and RT, and the N stages of the tumors were relatively advanced, namely, N2b(2), N2c(2), and N3(3), when compared with the stages of our tumors. These differences might explain the high volume reductions in the diseased lymph node in their study, though a simple comparison of their data and ours is impossible because they did not provide the axial dimension of the diseased nodes.
Other factors might predict the pathologic result of planned post-RT neck dissection. Such factors include intranodal focal low density, intranodal calcification, the presence and extent of extracapsular spread, and the size of the lymph node or nodes. These were statistically significant predictors of surgical outcome of planned post-RT neck dissection in the same cohort (6). A caveat is that some pathologically negative neck specimens may actually harbor residual cancer that is not detected by the pathologist that may progress (7) if a neck dissection is not performed.
The false-positive potential is less troublesome because all patients with a relatively poor response to RT are likely to undergo neck dissection until metabolic imaging findings can confidently exclude disease. 2-[Fluorine-18]fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) will likely be the first study to show sufficient precision for decision making in this regard. In fact, FDG-PET or a similar PET-based evaluation may ultimately replace most CT and/or MR imaging examinations in determining the necessity of post-RT neck dissection. However, low-volume (<1 cm3) residual tumor might be a limitation that produces false-negative results even when the metabolic technique is used.
In summary, the results of this study showed that the percentage decrease ratio of the largest axial dimension of the largest node in each hemineck is not a significant predictor of the pathologic status of the neck specimen after planned post-RT neck dissection. However, a hemineck with a decrease ratio greater than 50% tended to have a negative surgical specimen.
References
- 1.Parsons JT, Mendenhall WM, Cassisi NJ, Stringer SP, Million RR. Neck dissection after twice-a-day radiotherapy: morbidity and recurrence rates. Head Neck 1989;11:400–404 [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int Radiat Oncol Biol Phys 1986;12:733–740 [DOI] [PubMed] [Google Scholar]
- 3.Mabanta SR, Mendenhall WM, Stringer SP, Cassisi NJ. Salvage treatment for neck recurrence after irradiation alone for head and neck squamous cell carcinoma with clinically positive neck nodes. Head Neck 1999;21:591–594 [DOI] [PubMed] [Google Scholar]
- 4.Boyd TS, Harai PM, Tannehill SP, et al. Planned postradiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck 1998;20:132–137 [DOI] [PubMed] [Google Scholar]
- 5.Labadie RF, Yarbrough WG, Weissler MC, Pillsbury HC, Mukherji SK. Nodal volume reduction after concurrent chemo- and radiotherapy: Correlation between initial CT and histopathologic findings. AJNR Am J Neuroradiol 2000;21:310–314 [PMC free article] [PubMed] [Google Scholar]
- 6.Ojiri H, Mendenhall WM, Stringer SP, Johnson PL, Mancuso AA. Post-RT CT results as a predictive model for the necessity of planned post-RT neck dissection in patients with cervical metastatic disease from squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2002;52:420–428 [DOI] [PubMed] [Google Scholar]
- 7.Cummings BJ. Radiation therapy and the treatment of the cervical lymph nodes. In: Cummings CW, Fredrickson JM, Harker LA, et al. eds. Otolaryngology Head and Neck Surgery. 2nd ed. Vol 2. St Louis: Mosby–Year Book,1993;1626–1648