Thrombosis has emerged as a major concern in patients with coronavirus disease-2019 (COVID-19) (1,2). Most recently, thrombotic events, particularly cerebral venous sinus thrombosis (CVST) were reported within days after receiving severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) adenovirus-based vaccines (3). Initially, cases of CVST were reported in close succession to the use of the ChAdOx1 nCoV-19 vaccine (AstraZeneca), mostly in women of childbearing age, with many cases being associated with thrombocytopenia. Subsequently, 6 cases of CVST were reported in the United States with another adenovirus-based vaccine, Ad26.COV2.S vaccine (Johnson & Johnson), among women of childbearing age. CVST is a rare but potentially devastating disease (4). Therefore, this issue led to a temporary pause in use of the J&J vaccine in the United States and variable age-/sex-based restrictions for the AstraZeneca vaccine in other countries. Herein, we report the rate of CVST associated with these 2 vaccines based on publicly reported data, versus those occurring after COVID-19, and the estimated incidence rates in the US population.
We used the data from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) (5), and the US Centers for Disease Control and Prevention (CDC) (6) to report the number of events per vaccinated people with AstraZeneca and J&J vaccines, respectively. We used the data from a multinational study of cerebrovascular events to report CVST rates in hospitalized patients with COVID-19 (5). Data from the Nationwide Inpatient Sample database (an all-payer database including an approximate 20% sample of inpatient hospitalizations in the United States) from March and April 2018, the latest year with available information, were used to report the weighted monthly incidence of CVST with the use of principal discharge diagnostic codes 437.6 and 325 (with positive predictive value of 92% for CVST) (7) divided by the U.S. population as reported by the US census bureau. We estimated 99% confidence intervals (CIs) around the proportions.
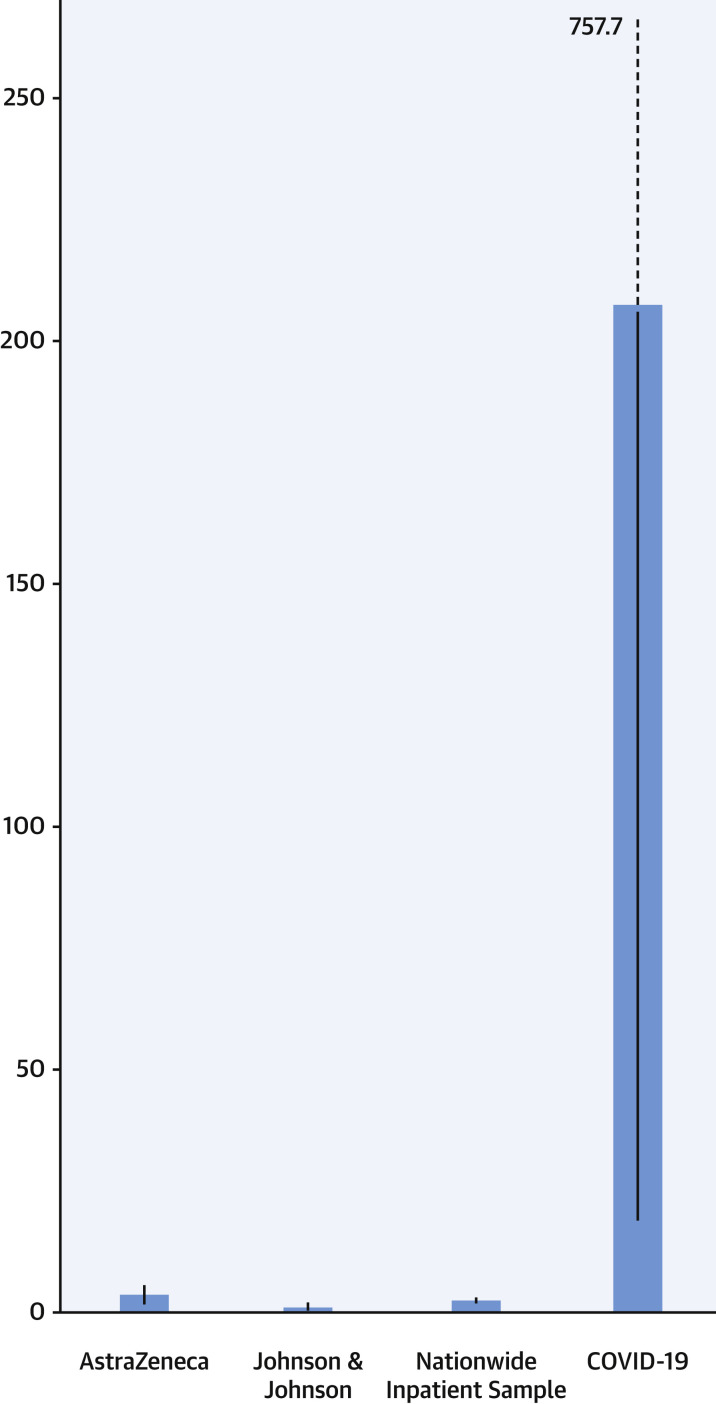
As of April 14, 2021, there were 77 CVST cases out of 21.2 million AstraZeneca vaccine recipients reported by the MHRA (3.6 per million; 99% CI: 2.7-4.8 per million). As of April 13, 2021, the CDC reported 6 cases of CVST out of 6.85 million vaccinated people (0.9 per million; 99% CI: 0.2-2.3 per million). In the Society of Vascular and Interventional Neurology COVID-19 registry, 3 out of 14,483 patients hospitalized with COVID-19 had CVST (207.1 per million; 99% CI: 23.3-757.7 per million). In the Nationwide Inpatient Sample, the weighted average rate of CVST in the US population for the months of March and April 2018 was 2.4 per million (99% CI: 2.1-2.6 per million) (Figure 1 ).
Figure 1.
Estimated Rate of CVST Per 1,000,000 People
Note the difference in event rates for CVST after AstraZeneca and Johnson & Johnson vaccinations, compared with event rates in hospitalized patients with COVID-19. Blue bars indicate the point estimates per million and the black lines indicate the estimated 99% confidence intervals. Note that the upper bound of the 99% confidence interval for patients hospitalized with COVID-19 was 757.7 per million. COVID-19 = coronavirus disease-2019; CVST = cerebral vein sinus thrombosis.
Discussion
The estimated relative frequency and 99% CI estimates for CVST for AstraZeneca and J&J vaccines based on the available reported events are markedly lower than those for patients hospitalized with COVID-19 and not substantially higher than monthly estimates from the US Nationwide Inpatient Sample. The estimates reported herein are in line with recommendations by regulatory authorities and indicative of the low absolute risk of CVST after adenovirus-based vaccination.
A unique feature of CVST after vaccination for SARS-CoV2 is the phenomenon of vaccine-induced thrombotic thrombocytopenia. This condition has features resembling heparin-induced thrombocytopenia, despite the absence of heparin exposure. The general consensus is to avoid heparin in these patients (3). Some experts recommend the administration of intravenous immunoglobulin or corticosteroids in patients with CVST associated with thrombocytopenia and antibodities against platelet factor 4 (8).
This study has several limitations. First, we attempted to provide relatively similar follow-up intervals. Nevertheless, the data sources have inherent differences. The AstraZeneca vaccine has not been authorized or used in the United States. Therefore, we report the events based on data from the MHRA in the United Kingdom. Although some regional variability due to biological, social, or health care–associated reasons is plausible, the event rates may be relevant to a broad audience. Second, the data from the Nationwide Inpatient Sample indicates the hospitalization rates, rather than incidence. However, most patients with CVST will require hospitalization for the initial stay. Third, the events are subject to underreporting, especially for cases for CVST after vaccination against SARS-CoV-2. The current analyses included updated estimates of these events. As additional events accrue over time, the estimated event rates will be affected. Fourth, we were unable to report event rates per specific age groups, because the numerators and denominators for such analyses are not publicly available from all the 4 data sources used in this paper. Fifth, the surveillance reports from the CDC and the MHRA do not provide sufficient details to understand the event rates for CVST associated with thrombocytopenia (so called vaccine-induced thrombotic thrombocytopenia). Sixth, the data used to estimate the CVST event rates in hospitalized patients with COVID-19 may be limited by voluntary inclusion of some hospitals in the registry (5). However, the study was multicenter and conducted from several countries. Even if some variation is expected across centers, the magnitude of risk for CVST appears to be several-fold higher in hospitalized patients with COVID-19 than in other groups. Finally, we were not able to report CVST with mRNA-based vaccines.
In conclusion, CVST is rare in the general population and after adenovirus-based SARS-CoV-2 vaccination, but appears to be several-fold more common in hospitalized patients with COVID-19. Additional research is required to fully elucidate the event rates, to understand the risk factors for vaccine-associated CVST and to identify strategies to prevent it. In the meantime, transparent realistic communication of the risk estimates will be helpful for shared decision making between patients and clinicians.
Funding Support and Author Disclosures
Dr Bikdeli is a consulting expert, on behalf of the plaintiff, for litigation related to 2 specific brand models of inferior vena cava filters. Dr Monreal has served as an advisor or consultant for Sanofi, Leo Pharma, and Daiichi-Sankyo; and has received a nonrestricted educational grant from Sanofi and Bayer to sponsor the Computerized Registry of Patients With Venous Thromboembolism. Dr Jimenez has served as an advisor or consultant for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Leo Pharma, Pfizer, ROVI, and Sanofi; has served as a speaker or a member of a speaker bureau for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Leo Pharma, ROVI, and Sanofi; and has received grants for clinical research from Daiichi-Sankyo, Sanofi, and ROVI. Dr Krumholz has received personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, Siegfried & Jensen Law Firm, Arnold & Porter Law Firm, Ben C. Martin Law Firm, and the National Center for Cardiovascular Diseases, Beijing, ownership of HugoHealth and Refactor Health, contracts from the Centers for Medicare and Medicaid Services; and has received grants from Medtronic, Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information; and is a Venture Partner at FPrime. Dr Goldhaber has received research support from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Boston Scientific, Daiichi-Sankyo, Janssen, the National Heart, Lung, and Blood Institute, and the Thrombosis Research Institute; and has received consulting fees from Bayer, Agile, Boston Scientific, and Boehringer Ingelheim. Dr Elkind has received royalties from UpToDate for chapters on stroke and COVID-19. Dr Piazza has received research grant support to Brigham and Women’s Hospital from EKOS, a BTG International Group company, Bayer, the Bristol Myers Squibb/Pfizer Alliance, Portola, and Janssen; and has received consulting fees from Amgen, Pfizer, Boston Scientific Corporation, and Thrombolex. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piazza G. Cerebral venous thrombosis. Circulation. 2012;125:1704–1709. doi: 10.1161/CIRCULATIONAHA.111.067835. [DOI] [PubMed] [Google Scholar]
- 5.The Medicines and Healthcare products Regulatory Agency (MHRA). Available at: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed April 25, 2021
- 6.Centers for Disease Control and Prevention Cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson & Johnson COVID-19 vaccine. April 13, 2021. https://emergency.cdc.gov/han/2021/han00442.asp Available at:
- 7.Siddiqui F.M., Weber M.W., Dandapat S., et al. Endovascular thrombolysis or thrombectomy for cerebral venous thrombosis: study of nationwide inpatient sample 2004-2014. J Stroke Cerebrovasc Dis. 2019;28:1440–1447. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Furie K.L., Cushman M., Elkind M.S.V., Lyden P.D., Saposnik G., American Heart Association/American Stroke Association Stroke Council Leadership Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021;52:2478–2482. doi: 10.1161/STROKEAHA.121.035564. [DOI] [PubMed] [Google Scholar]