Fig. 3. Prion-like spread of protein aggregates and proposed role of glymphatic transport.
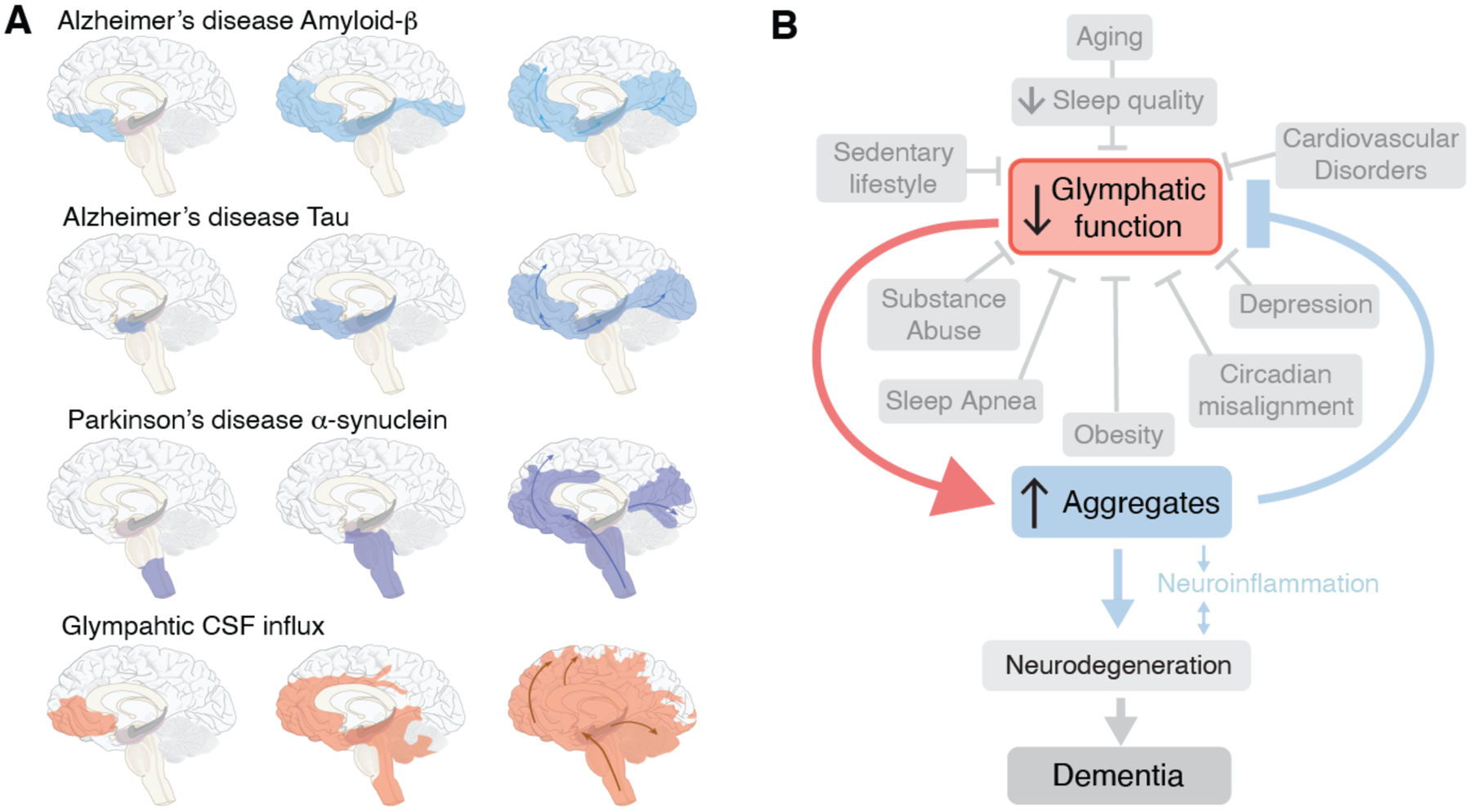
(A) Seeding and prion-like spread of protein aggregates (amyloid-ß and tau) in Alzheimer disease, and α-synuclein in Parkinson disease, relative to the distribution of glymphatic influx of a CSF tracer after intrathecal delivery (67). Prion-like spread of protein aggregates includes an extracellular component and thereby the possibility that the seeds are transported by the glymphatic system. (B) In this model, the glymphatic system resides at the intersection of a broad scope of disorders, which share an association with diminished brain fluid clearance. In addition, normal aging is also linked to a sharp decrease in the quality of sleep and in glymphatic flow. In turn, the stagnation of glymphatic flow, and hence that of extracellular proteins, contribute to protein aggregation, with misfolding and seeding, leading in turn to local inflammation, neuronal loss, and ultimately dementia.
