Figure 2. GluN1 deletion from GCs reduces mf-CA3 facilitation.
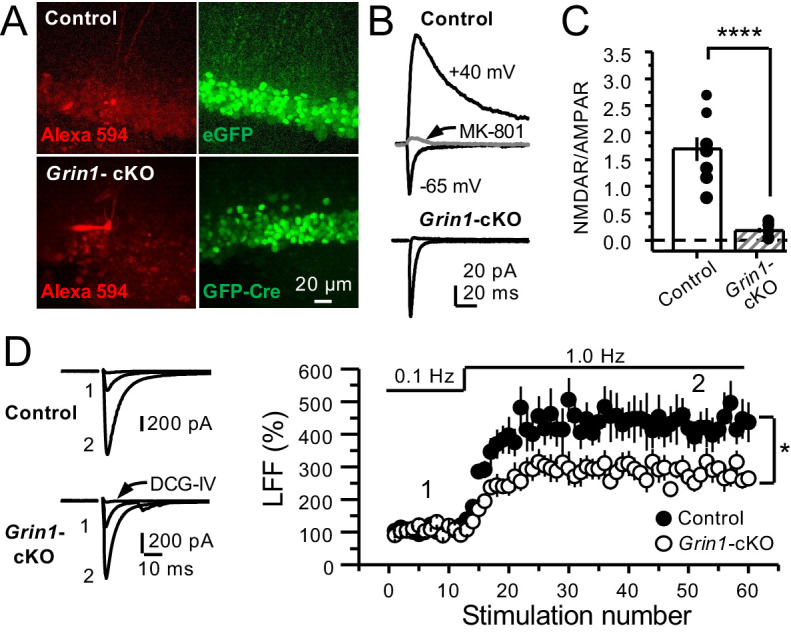
(A) Representative images showing GCs patch-loaded with Alexa 594 (35 µM) (left), and GFP expression in GCs (right). (B) Representative EPSCs recorded from control (GFP+) and Grin1-cKO (Cre-GFP+) GCs. Synaptic responses were elicited by activating medial perforant-path inputs. AMPAR-ESPCs were recorded at Vh = −65 mV in the presence of 100 µM picrotoxin, NMDAR-EPSCs were isolated with 10 µM NBQX and recorded at +40 mV. MK-801 (20 µM) was applied at the end of each recording. (C) Summary plot demonstrating that GluN1 deletion from GCs virtually abolished NMDAR-mediated transmission indicated by a strong reduction of NMDAR/AMPAR in Grin1-cKO GCs as compared to controls (control 1.61 ± 0.18, n = 9 cells, nine animals, Grin1-cKO 0.18 ± 0.04, n = 10 cells, 10 animals; control vs Grin1-cKO, p=9.2×10−6, unpaired t-test). (D) LFF was significantly reduced in GluN1-deficient animals (control, 430 ± 5%, n = 13 cells, 10 animals; Grin1-cKO, 291 ± 6%, n = 11 cells, 10 animals; p=0.0239, unpaired t-test). Representative traces (left) and summary plot (right). LFF was induced by stepping stimulation frequency from 0.1 to 1 Hz. DCG-IV (1 µM) was added at the end of each experiment. Data are presented as mean ± s.e.m. *p<0.05; ****p<0.001.
