To the Editor:
We read with interest the article: “Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) Vaccine: Causality or casualty?ˮ by Bril F. et al. 1 and the comment to the letter by Capecchi L. et al. recently published in J Hepatol.2
Although the onset of autoimmune hepatitis (AIH) in a young woman in the post-partum period, the presence of eosinophils at liver histology, and the short period elapsed after vaccine administration may support a coincidental association between AIH and SARS-CoV-2 vaccine,2 we recently observed a case of an 80-year-old woman who developed AIH 1 week after completing the schedule of Pfizer-BioNTech BNT162b2 mRNA vaccination. She was referred to our institution because of the recent onset of jaundice, hyperchromic urine, and elevated liver enzymes. The patient did not report smoking or alcohol use. Medical history included Hashimoto's thyroiditis on treatment with levothyroxine and an episode of acute glomerulonephritis in 1995 regressed after a cycle of corticosteroids. Current therapy was cholesterol-lowering pravastatin and aspirin for primary cardiovascular prevention. Physical examination was normal except for jaundice. Laboratories were significant for aspartate aminotransferase (AST) (1,401 IU/L; nl 0–40), alanine aminotransferase (ALT) (1,186 IU/L; nl 0–40) bilirubin (total 10.5 mg/dl; direct 7.5 mg/dl), alkaline phosphatase (ALP) (243 IU/L; nl 30-120) and gamma-glutamyl transferase (GGT) (524 IU/L; nl 7-44).
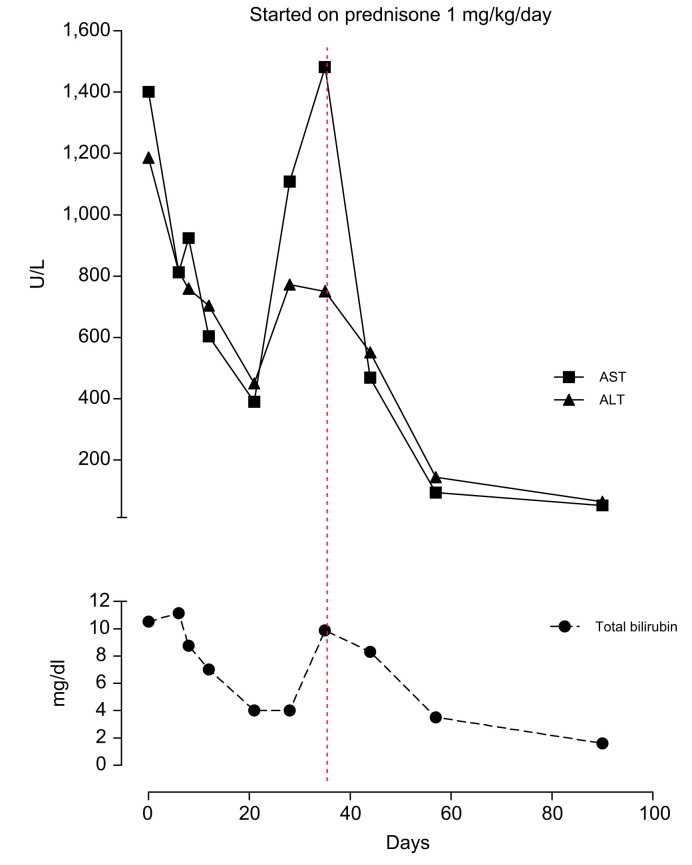
Hepatitis A, B, and C virus markers and HIV, Cytomegalovirus, Epstein Barr, Herpes simplex type 1 and 2 serology were negative. Ceruloplasmin and α1-antitrypsin levels were normal. The anti-mitochondrial, anti-smooth muscle, anti-liver-kidney microsomal antibodies were negative, while the antinuclear antibody (ANA) was positive (1:160, speckled pattern). Total IgG was 3,500 mg/dl (normal range: 750–1,600 mg/dl). Abdominal ultrasound showed enlarged reactive hilar lymph nodes (diameter up to 31 mm). Interface hepatitis with a moderate degree of lymphoplasmacytic infiltrate and multiple confluent foci of lobular necrosis with Councilman bodies were found at liver histology. According to the International Autoimmune Hepatitis Group (IAIHG) criteria,3 the patient's pre-treatment score was 19. The patient started a treatment schedule with prednisone at a dose of 1 mg/kg/day and subsequent tapering of 10 mg/week with a progressive improvement of the laboratory tests (Fig. 1 ).
Fig. 1.
Trends of plasma ALT, AST, and total bilirubin over time.
ALT, alanine aminotransferase; AST, aspartate aminotransferase. (This figure appears in color on the web.)
Several pathogens, such as Epstein–Barr, varicella-zoster, and hepatitis A viruses, can trigger AIH onset.4 In addition, some reports described a relationship between vaccination (i.e., hepatitis A and influenza virus) and the development of AIH,[5], [6], [7], [8] suggesting a potential role of both virus and vaccine in unmasking AIH in predisposed individuals. Thus, the occurrence of acute or chronic liver disease following viral infection or vaccination should raise the suspicion of AIH in the presence of other autoimmune disorders.
Although the causal link between the SARS-CoV-2 vaccine and AIH cannot be definitively established, our case report suggests that this association could be more than coincidental. Indeed, the medical history negative for liver disease as well as the coexistence of another autoimmune disorder, the reasonable lag time between exposure to the triggering factor, the typical onset of symptoms, the laboratory/histopathological findings and finally the excellent response to therapy are all pieces of the puzzle that reinforce the hypothesis of an association between AIH and SARS-CoV-2 vaccination.
In summary, since the vaccination campaign against SARS-CoV-2 is reaching extraordinary coverage rates, healthcare providers should be aware of the potential association between the vaccine and the onset of immunomediated disorders in patients with a history of autoimmune diseases.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Alba Rocco, Costantino Sgamato and Debora Compare: patient care, writing of the manuscript, and revision of the final version of the manuscript. Gerardo Nardone made a critical revision of the letter to the Editor.
Conflict of interest
Alba Rocco, Costantino Sgamato, Debora Compare declare no conflict of interest.
Gerardo Nardone has served as a speaker for Malesci and Takeda, and has received research funding from SOFAR Spa and Alfasigma.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.05.038.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021 Jul;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capecchi P.L., Lazzerini P.E., Brillanti S. Comment on “Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) Vaccine: Causality or casualty?”. J Hepatol. 2021 May doi: 10.1016/j.jhep.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez F., Berg P.A., Bianchi F.B., Bianchi L., Burroughs A.K., Cancado E.L., et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999 Nov;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 4.Zachou K., Muratori P., Koukoulis G.K., Granito A., Gatselis N., Fabbri A., et al. Review article: autoimmune hepatitis -- current management and challenges. Aliment Pharmacol Ther. 2013 Oct;38(8):887–913. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 5.Perumalswami P., Peng L., Odin J.A. Vaccination as a triggering event for autoimmune hepatitis. Semin Liver Dis. 2009 Aug;29(3):331–334. doi: 10.1055/s-0029-1233537. Epub 2009 Aug 12. PMID: 19676005. [DOI] [PubMed] [Google Scholar]
- 6.Berry P.A., Smith-Laing G. Hepatitis A vaccine associated with autoimmune hepatitis. World J Gastroenterol. 2007 Apr 21;13(15):2238–2239. doi: 10.3748/wjg.v13.i15.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki T., Suzuki Y., Ishida K., Kakisaka K., Abe H., Sugai T., et al. Autoimmune hepatitis following influenza virus vaccination: two case reports. Medicine (Baltimore) 2018 Jul;97(30) doi: 10.1097/MD.0000000000011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gemeren M.A., van Wijngaarden P., Doukas M., de Man R.A. Vaccine-related autoimmune hepatitis: the same disease as idiopathic autoimmune hepatitis? Two clinical reports and review. Scand J Gastroenterol. 2017 Jan;52(1):18–22. doi: 10.1080/00365521.2016.1224379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.