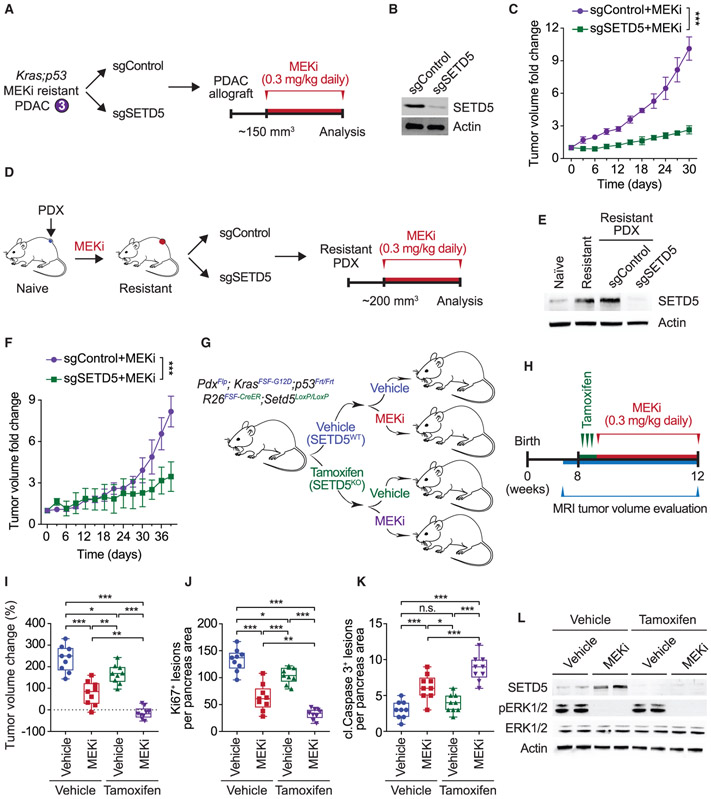
Figure 2. SETD5 Depletion Re-sensitizes Resistant PDAC to Trametinib in PDX and Mouse Models In Vivo.
(A) Schematic of generation of PDAC allografts in syngeneic mice established from MEKi-resistant tumor biopsies (as in Figure 1F) ± SETD5. Trametinib treatment schedule (MEKi, 0.3 mg/kg by intraperitoneal injection once daily) is shown.
(B) Western blots with the indicated antibodies of a representative sample for each condition described in (A) are shown. Actin is shown as a loading control.
(C) SETD5 depletion re-sensitizes PDAC allografts to MEKi. Quantification of mouse allograft tumor volume growth in syngeneic mice (n = 8 mice, for each treatment group). *p < 0.033, **p < 0.002, ***p < 0.0001 by two-way ANOVA with Tukey’s testing for multiple comparisons. Data are represented as mean ± SEM.
(D) Schematic to generate MEKi-resistant primary human PDAC for PDX studies. Patient tumor samples were grafted subcutaneously to immunocompromised NSG mice. Once tumor volume reached 200 mm3, mice were treated with trametinib 0.3 mg/kg by intraperitoneal injection once daily until tumor growth relapsed (~5 weeks), indicating drug resistance. Resistant cells were modified to express Cas9/sgRNA targeting SETD5 (sgSETD5) or control (sgControl) and tested for xenograft growth with treatment as shown.
(E) Western blots with the indicated antibodies of PDX samples in different stages described in (D). A representative sample for each condition is shown. Actin is shown as a loading control.
(F) SETD5 depletion restores refractory PDAC PDX tumor sensitivity to MEKi. Tumor volume quantification of MEKi-resistant patient-derived PDAC xenografts described in (D) in immunocompromised mice (n = 8 mice, for each treatment group). *p < 0.033, **p < 0.002, ***p < 0.0001 by two-way ANOVA with Tukey’s testing for multiple comparisons. Data are represented as mean ± SEM.
(G) Schematic of dual-recombinase (Flp/Frt, Cre/LoxP) system to acutely delete SETD5 in vivo in aggressive PDAC. Activation of KrasFSF-G12D and deletion of p53Frt/Frt alleles in mouse pancreata (Pdx1Flp) results in development of malignant PDAC. Time-specific tamoxifen-mediated Rosa26FSF-CreERT2 activation allows for recombination of the conditional Setd5LoxP/LoxP allele with loss of SETD5 expression (SETD5KO) in established PDAC. Control animals that received vehicle express wild-type SETD5WT. Subsequently mice were treated with placebo (vehicle) or trametinib (MEKi, 0.3 mg/kg, intraperitoneal injection once daily).
(H) Treatment schedule for administration of tamoxifen, MEKi or placebo (vehicle) in the system described in (G).
(I) Deletion of SETD5 in established PDAC cooperates with MEKi to suppress tumor growth. Quantification of PDAC volume change based on MRI scans (detailed procedure in the STAR Methods) in mice described in (G and H) (n = 9 mice for each experimental group). Boxes, 25th to 75th percentiles; whiskers, minimum to maximum; center line, median; *p < 0.033, **p < 0.002, ***p < 0.0001 by two-way ANOVA with Tukey’s testing for multiple comparisons. Scale bars, 100 μm.
(J and K) (J) Quantification of proliferation (Ki67+ cells), and (K) cleaved caspase-3 (cl.Caspase3+ cells) a marker of apoptosis in samples as in (I). Boxes, 25th to 75th percentile; whiskers, minimum to maximum; center line, median; arrowheads, positive cleaved caspase-3 cells; *p < 0.033, **p < 0.002, ***p < 0.0001 by two-way ANOVA with Tukey’s testing for multiple comparisons. Data are represented as mean ± SEM. Scale bars, 100 μm.
(L) Western blots with the indicated antibodies of the indicated pancreatic tissue lysates. Two independent and representative samples are shown for each genotype. Actin is shown as a loading control.
See also Figure S2.