Abstract
Models of drug addiction in rodents are instrumental in understanding the underlying neurobiology. Intravenous self-administration of drugs in mice is currently the most commonly used model; however, several challenges exist due to complications related to catheter patency. To take full advantage of the genetic tools available to study opioid addiction in mice, we developed a non-invasive mouse model of opioid self-administration using vaporized fentanyl. This model can be used to study various aspects of opioid addiction including self-administration, escalation of drug intake, extinction, reinstatement, and drug seeking despite adversity. Further, this model bypasses the limitations of intravenous self-administration and allows the investigation of drug taking over extended periods of time and in conjunction with cutting-edge techniques such as calcium imaging and in vivo electrophysiology.
Keywords: Vapor, Fentanyl, Self-administration, Mouse model, Opioid addiction, Opioid use disorder
Background
Vapor inhalation is emerging as an alternative route of drug self-administration (instead of intravenous) to study addiction to opioids and other drugs. This has been accomplished in rats using alcohol (Vendruscolo and Roberts, 2014; de Guglielmo et al., 2017 ), nicotine ( Smith et al., 2020 ), cannabis ( Freels et al., 2020 ; Muthusamy, 2020), and opioids such as sufentanil ( Vendruscolo et al., 2018 ), fentanyl ( McConnell et al., 2020 ), and heroin ( Gutierrez et al., 2020 ). We recently adapted this model and developed a mouse model of opioid vapor self-administration using fentanyl ( Moussawi et al., 2020 ), the protocol for which is described in this manuscript.
Protocol overview: Mice (males and females) are trained to self-administer fentanyl vapor for 1-h sessions, during which each response on the active operandum (active lever press or nosepoke into the active port) triggers a vapor delivery on a fixed-ratio 1 (FR1) schedule of reinforcement (this can be adjusted to higher FRs, e.g., 3, 5, or 10) (Video 1). Each vapor delivery is followed by a timeout period of 60 s, during which additional responses on the operandum are recorded but do not result in additional drug delivery. The timeout period is signaled by a cue light that is turned on and remains on for the duration of the timeout. Operant activity on an inactive lever/nosepoke port is recorded but has no consequences. After reaching stable pressing (<25% variation in the number of vapor deliveries relative to the average vapor deliveries of the 3 preceding sessions; usually 5-8 sessions, at least 24-h apart), mice are matched based on their operant response and split between short- (ShA) and long-access (LgA) groups. ShA mice are allowed to self-administer fentanyl vapor for 1 h every other day, whereas LgA mice are allowed to self-administer fentanyl for 6 or 12 h every other day. Escalation of fentanyl intake is typically observed over 8-10 sessions in the LgA group, whereas response in the ShA group remains stable.
Video 1. Video showing a mouse self-administering fentanyl vapor.

Note the increased locomotion caused by fentanyl intake and the straub tail reaction after vapor delivery. Reprinted/adapted from Moussawi et al. (2020) . © The Authors, some rights reserved; exclusive licensee, American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.
Materials and Reagents
-
Animals
Adult (male and female, 8-10 weeks old) C57BL/6J mice are purchased from JAX laboratories (Bar Harbor, ME, USA). The mice are allowed to habituate in the animal facility for a week. Mice are group-housed and maintained on a 12 h reverse light/dark cycle in a room with a controlled temperature (22 ± 2°C) and humidity (50%). Mice have free access to water and food in their home cages. Training sessions are performed during the dark cycle, and the body weight is recorded at least once a week.
Fentanyl citrate (NIDA Drug Supply Program, Bethesda, MD, USA)
Vegetable glycerin (Essential Elements, Boulder, CO, USA, available on Amazon; UPC code: 703610139756)
Propylene glycol (MP Biochemicals, catalog number: 151957)
Capsaicin (AK Scientific, catalog number: N735-5g)
Ethanol 200 proof (Pharmco-Aaper, catalog number: 111000200)
Narcan kit (available at pharmacies)
Equipment
-
Chambers
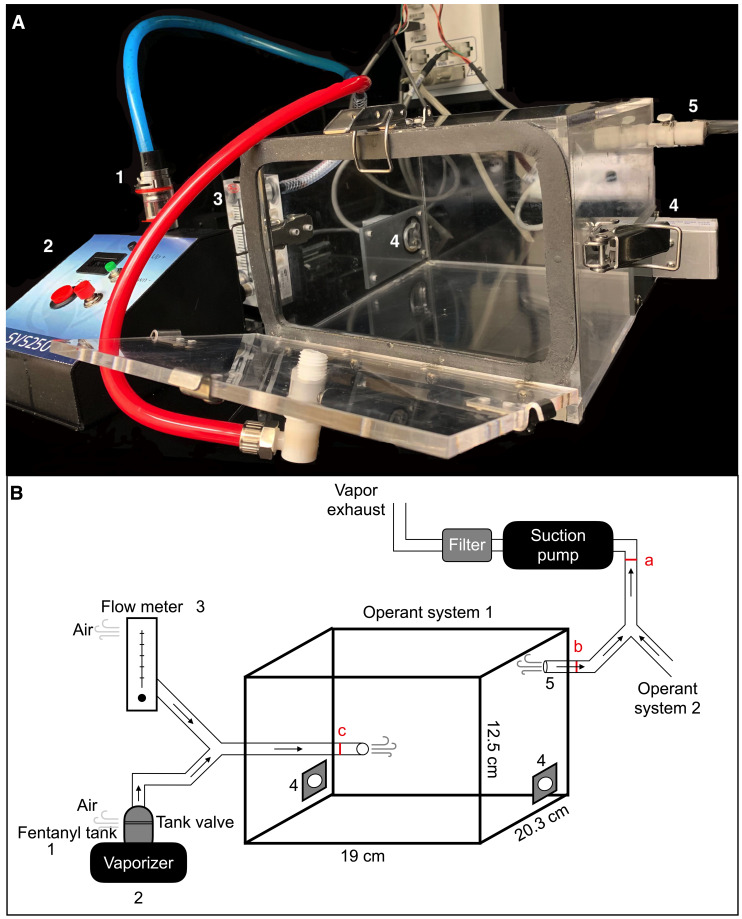
Airtight chambers are customized to order and made from transparent plexiglass (Figure 1) (La Jolla Alcohol Research, La Jolla, CA, USA). These can be nosepoke or lever press chambers.
-
Nosepoke chambers
Two nosepoke ports (1 cm in diameter) are mounted opposite to each other on the side walls, 1.5 cm from the floor. White light bulbs are mounted inside the nosepoke ports. The vapor delivery port is mounted in one of the walls or in the door of the chamber, 7 cm from the chamber floor. The exhaust (suction) port is mounted in one of the walls, preferably opposite the vapor delivery port, 12 cm from the chamber floor.
Note: You can substitute nosepoke chambers for lever chambers: the inner and outer dimensions are the same. The levers and cue lights are installed in the right and left walls. The levers are positioned 1.5 cm from the floor, and the cue lights 6.5 cm from the floor. The vapor delivery and exhaust ports are positioned similarly to the nosepoke chambers.
-
Chamber enclosure
The chambers are placed in a black Plexiglas enclosure to minimize noise and light. The enclosures can fit four or eight individual chambers, depending on the size of the enclosure.
Vaporizer: SVS250 vaporizer (Scientific Vapor, OR, USA)
Drug solution tanks: TFV8 X-baby Smok tanks, 4 ml (Shenzhen IVPS Technology, catalog number: TC005301000)
Coils for the tanks: V8 X-Baby Q2, 0.4 Ω dual coils (Shenzhen IVPS Technology, catalog number: TA104301000)
-
Exhaust system
The goal of this system is to generate negative pressure in the operant chamber, which drives vapor into the chamber and then clears it out: an air compressor pump (75 DG model HK-25L; Hakko, catalog number: E307612) that generates vacuum suction is connected to the chamber on one end, and to an inline disposable HEPA-Cap filter (Whatman 6702-3600 hepa-Cap 36, Cole Parmer, catalog number: EW-29700-92) on the other end (Figure 1B). The tubing from the HEPA filter is then connected to the facility’s exhaust system.
Air flow controller (Dwyer, model: VFA-23-SSV)
-
Med-associates hardware (Med Associates, Fairfax, VT, USA):
DIG-700G (decode card – PCI)
DIG-704PCI-2 (interface card – PCI)
SG-6080D (small tabletop cabinet + power supply (120 V/60 Hz))
SG-210CB (DB-25 smart control cable, M/F, 25' (7.6 m)
DIG-716 (SmartCtrlTM interface module, 4 in/8 out)
SG-716 (SmartCtrlTM connection panel, 4 in/8 out)
Figure 1. Vapor chamber setup.
A. Different components of the vapor chamber apparatus: (1) tank where the fentanyl solution is loaded; (2) vaporizer; (3) flow meter; (4) nosepoke ports on opposite walls; and (5) exhaust port that connects to the vacuum suction pump. Photo credit: Maria Ortiz, NIDA. B. Sketch of the vapor chamber setup including airflow dynamics, suction pump, and HEPA filter. The numbers in B correspond to those in A. Inner dimensions of the vapor chamber are indicated in cm; the internal volume of the chamber is ~5 L. The pump creates negative pressure that determines the airflow rate in the chambers. The total negative pressure generated by the pump is measured by connecting a flow meter between the pump and the tubing from all the chambers – red bar a (~16 L/min). We measure the total airflow in each chamber by connecting a flow meter between the exhaust port and the tubing from the pump – red bar b (4 L/min). To check whether the chamber is airtight, we measure the airflow between the inlet port and the tubing from the vaporizer – red bar c; if this does not match the total airflow from the chamber measured near the exhaust port (at red bar b), the chamber is not airtight and the leak must be addressed. Reprinted/adapted from Moussawi et al. (2020) . © The Authors, some rights reserved; exclusive licensee, American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.
Software
MedPC (Med Associates, Fairfax, VT, USA)
GraphPad Prism (Version 8, GraphPad Software, San Diego, CA, USA)
Procedure
-
Before starting the experiment
-
Preparing and handling of the fentanyl solution
When handling fentanyl in any form (powder, solution, or vapor), wear personal protective equipment (PPE) including gloves, gown, mask, and eye shield because fentanyl is highly lipophilic and easily absorbed through the skin and mucosal membranes. Post a note on the door of the rooms where fentanyl is handled, indicating the requirement for full PPE and a reminder not to touch any object in the room without gloves to avoid accidental exposure. The door of the experimental room where vapor self-administration is conducted should be closed. We recommend having a Narcan kit available in the unlikely case of accidental exposure and fentanyl toxicity by staff.
Fentanyl stock solution (20 mg/ml): Prepare a stock solution of 80% vegetable glycerin (VG, 400 ml) and 20% propylene glycol (PG, 200 ml). Add 6 ml sterile water to 1 g fentanyl. Vortex the solution, then add 44 ml VG/PG stock. Sonicate in a warm bath at 50°C for 1 h or until the fentanyl is completely dissolved. Vehicle control solution is VG/PG (80/20).
Fentanyl experimental solution for self-administration (5 mg/ml): Dilute the stock solution with VG/PG to the desired concentration. For a solution of 5 mg/ml, mix 5 ml stock solution with 15 ml VG/PG (total 20 ml fentanyl at 5 mg/ml).
Capsaicin-adulterated fentanyl: Prepare a stock solution of capsaicin dissolved in 100% ethanol at 10 mg/ml (10 mg in 1 ml). Capsaicin is a potent irritant; we recommend weighing it and preparing the stock solution in a fume hood using full PPE. Add 0.04 ml capsaicin stock solution to 20 ml pre-prepared 5 mg/ml fentanyl solution (0.2% capsaicin). Depending on the experimental setup and flow dynamics of the vapor within the chambers, one may need to adjust the capsaicin concentration (0.1-0.3%) to ensure that it is not high enough to cause complete avoidance of the active lever but strong enough to significantly suppress drug self-administration. The optimal concentration of capsaicin for a given experiment can be determined in a pilot cohort of mice trained under FR1 in 1-h sessions (short access) until they reach a stable response for 3 consecutive sessions. Allow the mice to self-administer fentanyl-adulterated capsaicin for two 1-h sessions. The goal is to have a 50-75% reduction in vapor deliveries in the second session ( Moussawi et al., 2020 ). If the number of vapor deliveries declines to zero in the second session, then the capsaicin concentration is too high and needs to be lowered. In this case, a long-lasting adverse effect may occur, in that mice do not return to baseline self-administration levels following an exposure to capsaicin.
-
Calibrating the vapor chambers
Vaporizing the VG/PG solution with or without fentanyl results in a dense white vapor cloud. We calibrate our system to result in vapor that persists in the chamber for no more than 1 min after each vapor delivery. The small size of the chamber and the vaporization settings allow for homogenous distribution of the vapor throughout the chamber and removal within a minute.
The duration of vapor exposure in the chamber depends mainly on the interplay of 3 adjustable factors: 1) vaporization time: we use 1.5-3 s; 2) vaporizer power setting: we usually set it at 60 W (as recommended for the particular type of coil that we use); and 3) air-flow in the chamber. Air-flow depends mainly on the power of the suction pump. A pump may be connected to more than one chamber simultaneously, provided that it produces adequate air-flow. The air-flow through each chamber, assuming that the chamber is airtight, enters the system through two entry points: 1) the flow meter; and 2) the tank valve (we keep it in the open position) (Figure 1). The sum of the air-flow through these two paths (measured by connecting a flow meter at red bar c, in Figure 1B) is equivalent to the total flow through the chamber (measured by connecting a flow meter at red bar b, in Figure 1B; this was around 4 L/min/chamber in our setup). Air-flow in each of the two paths (flow meter and tank valve) negatively affects flow through the other path; therefore, to increase or decrease the flow of vapor into the chamber, we decrease or increase the flow through the flow meter, respectively. We like the air-flow through the tank to be around 2 L/min, so we set the flow meter to 2 L/min (total flow through the chamber = 4 L/min).
To ensure that the chamber is airtight, the measured flow at red bar b must be equal to the flow at red bar c (Figure 1B); otherwise, this indicates that the chambers are not airtight and the vapor flow is compromised. This can result from loose connections, damaged sealing strips in the chamber door, loose screws, or a break in the Plexiglass. We recommend performing the calibration weekly to minimize variability in drug exposure across sessions.
-
Preparing the operant procedure program
All operant procedures are programed through the MedPC software. Set the appropriate parameters needed for the experiment: cues, timeout, duration of vaporization, and schedule of reinforcement (FR 1, 3, 5, etc.). In our experiments, we predominantly used an FR1 schedule where each active nosepoke or lever press triggers the vaporizer for 1.5 s (set at 60 W), resulting in vapor delivery (Figure 2). For self-administration data at higher FR schedules, please refer to the original manuscript ( Moussawi et al., 2020 ). Each vapor delivery is followed by a timeout period of 60 s, during which the cue light is turned on. Presses during timeout are recorded but have no consequences. The timeout period is determined based on the clearance time of fentanyl vapor from the chamber. Inactive lever presses or nosepokes are recorded but have no programmed consequences.
For the first behavioral session, we set a limit for the maximum number of vapor deliveries (~5) to avoid excessive fentanyl exposure, since the mice randomly explore the chambers without having learned the experimental contingencies.
For the first three long-access sessions, we also limit the maximum number of vapor deliveries (~30/12 h), since mice tend to binge on fentanyl vapor in the first couple of long-access training sessions ( Moussawi et al., 2020 ).
Optional: Habituate the mice to the operant chambers by simply placing them in the operant chambers for 30-60 min prior to starting the acquisition sessions. Active lever presses or nosepokes do not have programmed consequences during habituation sessions.
-
-
Fentanyl vapor self-administration protocol
Turn on the MedPC interface cabinet and computer.
Fill the tanks with the fentanyl or vehicle solution. Use separate tanks for the vehicle, fentanyl, and capsaicin-adulterated fentanyl.
Screw the tanks onto the vaporizers.
Close the doors of all chambers.
-
Test all the chambers to make sure that the fentanyl/vehicle is properly vaporized and the air-flow is homogenous throughout a chamber and between chambers. This can be performed either by pressing the red ‘fire’ button on the vaporizer for about 1 s or through a pre-programmed MedPC program.
If no vapor is flowing: Calibrate the chambers again to determine whether there are air-flow-related issues, e.g., leak in a chamber (see potential reasons above in Step A2 calibration section), tank lid not closed, loose connections, tubing clogged with condensed vapor, or a saturated HEPA filter. Address the problem accordingly to resolve the issue.
Other possibilities: The tank coil is burned out causing a failure to vaporize the solution, the tank is out of drug, or the vaporizer is not recognizing the coil automatically.
Note: Vapor chambers should not be opened until all the vapor has been cleared.
Load the MedPC self-administration program.
Line the chamber floors with cotton pads (Alpha pads; Animal Specialties and Provisions, Quakertown, PA, USA) to absorb urine.
-
Weigh the animals and place them in their corresponding chamber.
Use separate chambers for fentanyl vs. vehicle mice (recommended).
Use separate chambers for male vs. female mice (recommended).
For longer sessions, provide food and water in the chambers using food cups and sipper bottles on the floor.
Close all the latches.
Close the chamber enclosure.
-
Start the self-administration session.
If mice are not readily acquiring self-administration behavior after 2 acquisition sessions, especially in chambers with lever presses instead of nosepokes, a small smear of peanut butter can be added to the active nosepoke port or lever ( Towers et al., 2019 ) to encourage operant responses. Stop the use of peanut butter once the mice have responded on the active operandum.
-
At the end of the session, remove the mice from the operant chambers and place them back in their home cages. Discard the cotton pads and clean the chambers thoroughly.
Use water-base cleaning solutions that are compatible with Plexiglass.
Thoroughly clean the tubing (air duster cans or air compressor) and chambers after each capsaicin session, since trace amounts of capsaicin may be sufficient to disrupt behavior.
Discard anything contaminated with fentanyl (i.e., gloves, paper towels, cotton pads, etc.) in Medical Pathological Waste (MPW) boxes.
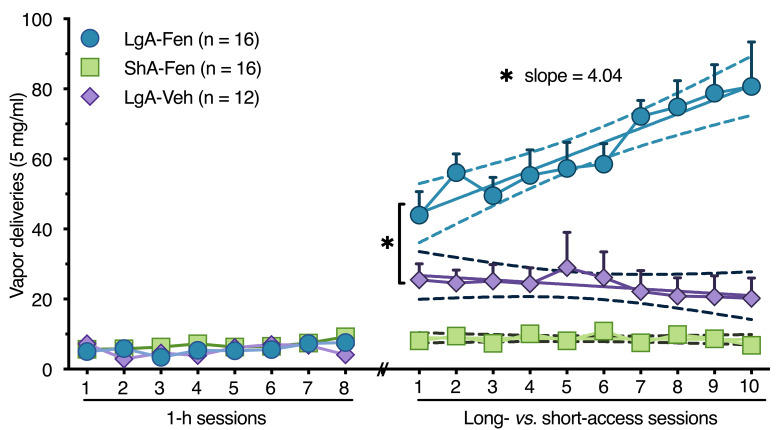
Figure 2. Example data showing escalation of fentanyl vapor self-administration.
Mice escalate fentanyl vapor self-administration during long-access sessions (LgA-Fen). Linear regression analysis showing a positive slope for the LgA-Fen group (a = 4.04, CI: 2.46-5.62), which is significantly greater than 0 (F(1, 158) = 25.53, r2 = 0.14, P < 0.0001). The slopes for the short-access fentanyl (ShA-Fen) and long-access vehicle (lgA-Veh) groups were not different from 0 (a = -0.058, CI: -0.33 to 0.22 and a = -0.64, CI: -1.92 to 0.63, respectively), suggesting the absence of escalation in these groups. Data are expressed as the mean ± SEM, *P < 0.05. Dashed lines represent 95% confidence intervals. Reprinted/adapted from Moussawi et al. (2020) . © The Authors, some rights reserved; exclusive licensee, American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.
Data analysis
We analyzed and plotted data using GraphPad Prism (version 8, GraphPad Software, San Diego, CA, USA). We used t-tests (paired or unpaired) and analysis of variance (ANOVA; one- or two-way, with or without repeated measures) to compare different groups and operant responses to active vs. inactive operandi. The proper t-tests and ANOVAs were employed after confirmation of normal data distribution and homogeneity of variance using tests such as the Shapiro-Wilk and Levene homogeneity tests. We used Sidak’s or Bonferroni’s test for post-hoc comparisons, where appropriate. The escalation data were also analyzed using linear regression models to account for the unidirectional effect of time. To ensure that the model was appropriate for the data and there was no departure from linearity, we used the replicates test. Statistical significance was set at P ≤ 0.05. All data are expressed as the mean and standard error of the mean. The regression model is expressed as the mean and 95% confidence intervals (Figure 2).
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute for Drug Abuse, DA048085 (KM) and DA048530 (BJT), and by the Center for Compulsive Behaviors, National Institutes of Health via the NIH Director’s Challenge Award (RCNM). This protocol is based on the manuscript “Fentanyl vapor self-administration model in mice to study opioid addiction” ( Moussawi et al., 2020 ).
Competing interests
The authors declare no conflicts of interest.
Ethics
This protocol was performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Animal Care and Use Committee of the National Institute for Drug Abuse (Protocol # 17-CNRB-133).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. de Guglielmo G., Kallupi M., Cole M. D. and George O.(2017). Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology(Berl) 234(13): 2009-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freels T. G., Baxter-Potter L. N., Lugo J. M., Glodosky N. C., Wright H. R., Baglot S. L., Petrie G. N., Yu Z., Clowers B. H., Cuttler C., Fuchs R. A., Hill M. N. and McLaughlin R. J.(2020). Vaporized Cannabis Extracts Have Reinforcing Properties and Support Conditioned Drug-Seeking Behavior in Rats. J Neurosci 40(9): 1897-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutierrez A., Nguyen J. D., Creehan K. M. and Taffe M. A.(2020). Self-administration of heroin by vapor inhalation in female Wistar rats. bioRxiv. [Google Scholar]
- 4. McConnell S. A., Brandner A. J., Blank B. A., Kearns D. N., Koob G. F., Vendruscolo L. F. and Tunstall B. J.(2020). Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology 182: 108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moussawi K., Ortiz M. M., Gantz S. C., Tunstall B. J., Marchette R. C. N., Bonci A., Koob G. F., and Vendruscolo L. F.(2020). Fentanyl vapor self-administration model in mice to study opioid addiction. Sci Adv 6(32): eabc0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muthusamy A. K.(2020). Cannabis Extract Composition Determines Reinforcement in a Vapor Self-Administration Paradigm. J Neurosci 40(33): 6264-6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith L. C., Kallupi M., Tieu L., Shankar K., Jaquish A., Barr J., Su Y., Velarde N., Sedighim S., Carrette L. L. G., Klodnicki M., Sun X., de Guglielmo G. and George O.(2020). Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology 45(11): 1909-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Towers E. B., Tunstall B. J., McCracken M. L., Vendruscolo L. F. and Koob G. F.(2019). Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151: 189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vendruscolo J. C. M., Tunstall B. J., Carmack S. A., Schmeichel B. E., Lowery-Gionta E. G., Cole M., George O., Vandewater S. A., Taffe M. A., Koob G. F., and Vendruscolo L. F.(2018). Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacology 43(4): 801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vendruscolo L. F. and Roberts A. J.(2014). Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48(3): 277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]