Abstract
Background & Aims
The Liver Cancer Risk test algorithm (LCR1-LCR2) is a multianalyte blood test combining proteins involved in liver cell repair (apolipoprotein-A1 and haptoglobin), known hepatocellular carcinoma (HCC) risk factors (sex, age, and gamma-glutamyl transferase), a marker of fibrosis (alpha2-macroglobulin) and alpha-fetoprotein (AFP), a specific marker of HCC. The aim was to externally validate the LCR1-LCR2 in patients with chronic HCV (CHC) treated or not with antivirals.
Methods
Pre-included patients were from the Hepather cohort, a multicentre prospective study in adult patients with CHC in France. LCR1-LCR2 was assessed retrospectively in patients with the test components and AFP, available at baseline. The co-primary study outcome was the negative predictive value (NPV) of LCR1-LCR2 for the occurrence of HCC at 5 years and for survival without HCC according to the predetermined LCR1-LCR2 cut-offs. The cut-offs were adjusted for risk covariables and for the response to HCV treatment, and were quantified using time-dependent proportional hazards models.
Results
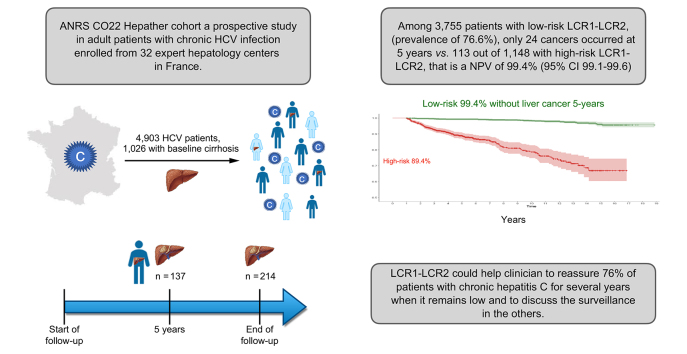
In total, 4,903 patients, 1,026 (21.9%) with baseline cirrhosis, were included in the study. Patients were followed for a median of 5.7 (IQR 4.2–11.3) years. A total of 3,788/4,903 (77.3%) patients had a sustained virological response. There were 137 cases of HCC at 5 years and 214 at the end of follow-up. HCC occurred at 5 years in 24/3,755 patients with low-risk LCR1-LCR2 compared with 113/1,148 patients with high-risk LCR1-LCR2. The NPV was 99.4% (95% CI 99.1–99.6). Similar findings (hazard ratio, 10.8; 95% CI, 8.1–14.3; p <0.001) were obtained after adjustment for exposure to antivirals, age, sex, geographical origin, HCV genotype 3, alcohol consumption, and type 2 diabetes mellitus.
Conclusions
The results showed that LCR1-LCR2 can be used to successfully identify patients with HCV at very low risk of HCC at 5 years.
Lay summary
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide and the fastest growing cause of cancer death in many countries. We constructed and internally validated a new multianalyte blood test to assess this Liver Cancer Risk (LCR1-LCR2). This study confirmed the performance of LCR1-LCR2 in patients with chronic HCV in the national French cohort Hepather, and its ability to identify patients at a very low risk of HCC at 5 years.
Clinical Trials registration
The study is registered at ClinicalTrials.gov (NCT01953458).
Keywords: Fibrosis progression, Cirrhosis, Multi-analyte blood test, LCR1-LCR2, Surveillance, AFP, FibroTest™, Liver Cancer Risk
Abbreviations: AFP, alpha-fetoprotein; AUROC, area under the receiver operating curve; CHC, chronic HCV; DAA, direct-acting antivirals; EASL, European Association for the Study of the Liver; FIB4, Fibrosis-4; HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk; NNS, needed to screen; NPV, negative predictive value; SIR, standardised incidence ratio; STARD, Standards for the Reporting of Diagnostic Accuracy Studies; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SVR, sustained virological response; VCTE, vibration-controlled transient elastography
Graphical abstract
Highlights
-
•
HCC is the fourth leading cause of cancer-related death worldwide and the fastest growing cause of cancer deaths in the USA.
-
•
The American Association for the Study of Liver Diseases recommends surveillance every 6 months only in patients with cirrhosis.
-
•
The LCR1-LCR2 algorithm is a multianalyte blood test combining proteins involved in cell repair, fibrosis and liver cancer.
-
•
The LCR1-LCR2 algorithm was able to identify patients with chronic HCV at very low risk of HCC at 5 years.
-
•
This algorithm could help clinicians to reassure a percentage of patients with chronic HCV that their risk of developing HCC remains low.
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide and the fastest growing cause of cancer death in the USA.1 The prognosis of HCC is poor except in the subset of patients who are diagnosed at an early stage of disease. The ongoing increase in the incidence of HCC shows the importance of effective surveillance strategies, especially in emerging at-risk cohorts, such as patients with chronic HCV (CHC) and a sustained virological response (SVR).[2], [3], [4]
In western countries, Egypt, and Japan, the main cause of HCC is HCV infection. Despite screening of baby-boomers,5,6 as well as the efficacy of direct-acting antivirals (DAA) on necroinflammatory activity, the progression of fibrosis, and occurrence of HCC,[7], [8], [9] the effectiveness of surveillance remains a matter of debate.1,3,4 The American Association for the Study of Liver Diseases only recommends surveillance every 6 months in patients with cirrhosis, based on ultrasound with or without serum alpha-fetoprotein (AFP), defined here as ‘standard surveillance’.2 The European Association for the Study of the Liver (EASL) recommends the same surveillance extended to the precirrhotic stages of fibrosis (F3).10
Criticisms of surveillance include a small net benefit and increased harm because of false-positive results.1,3,4 To increase the sensitivity without decreasing the specificity of surveillance, we recently constructed and internally validated the Liver Cancer Risk test algorithm (LCR1-LCR2) in patients from the Groupe-Hospitalier-Pitié-Salpêtrière in Paris, France (Assistance Publique Hôpitaux de Paris, FibroFrance-GHPS cohort), referred to here as the ‘Original Study’.11 LCR1 combines proteins involved in liver cell repair (apolipoprotein A1 and haptoglobin), known risk factors (sex, age, and gamma glutamyl transpeptidase) and a marker of fibrosis (alpha2-macroglobulin), with a very high negative predictive value (NPV) for the occurrence of HCC.11 Among patients with mixed causes of liver disease, the LCR1-LCR2 algorithm, which included AFP, had a 99.5% (99.0–99.7%) NPV at 5 years. LCR1-LCR2 has also been externally validated in a case-control study of 149 patients prospectively enrolled in the Bondy Cohort.12
The aim of the present study was to externally validate LCR1-LCR2 with the previously identified cut-offs of the Original Study, in a sustainability analysis of patients with CHC, prospectively included in the large Hepather cohort, regardless of the stage of fibrosis at inclusion.7
Patients and methods
Study design and participants
The ANRS CO22 Hepather cohort is a French national, multicentre, prospective, observational cohort study of patients with active or inactive HBV or past or present HCV infection, which started in August 2012.6 Here, adult patients with HCH infection were recruited consecutively during a medical visit at 1 of 32 expert hepatology centres in France. Patients with chronic HBV, a history of decompensated cirrhosis or liver transplantation, and patients who were treated with interferon-ribavirin with or without first-generation protease inhibitors were excluded. The main exclusion criteria were HIV co-infection and ongoing treatment for HCV infection at inclusion.
Written informed consent was obtained from each patient before enrolment. The protocol was performed in accordance with the Declaration of Helsinki and French law for biomedical research and was approved by the Comité-Protection-Personnes-Ile de France ethics committee (Paris, France) and the French Regulatory Authority (ANSM). The Hepather cohort has been previously described elsewhere.7
In the current post hoc analysis, we selected all patients with CHC infection at entry and with a reliable measurement of fibrosis using the FibroTest™ (BioPredictive, Paris, France), a validated biomarker of the stages of fibrosis before inclusion. The population of interest purposely included consecutive patients, treated, not treated, SVR and not SVR, to validate the biomarkers in these different subsets, as performed in the Original Study.
It was possible to assess the LCR1 value from the FibroTest™ components, thus identifying patients without cirrhosis but with a high risk of HCC, defined by the predetermined cut-off ≥0.0154. Despite the absence of cirrhosis, all of these at-risk patients as well as patients with cirrhosis defined by a FibroTest™ >0.74 who were already known to be at risk of HCC,1 were included if AFP was also available to assess LCR2.11 To perform and preserve the independence of an external validation, the patients of the Hepather cohort followed at the Pitié-Salpêtrière expert centre, where the LCR1-LCR2 was constructed and internally validated, were not included. This was an ambispective study,13 because patients were included prospectively, but if patients were missing components of LCR1-LCR2, those data were assessed retrospectively either when a frozen serum sample was available at inclusion or using the components of the previously performed routine FibroTest™.
Procedures
Blood and urine samples were obtained and stored in a centralised biobank (Cell & Co Biorepository, Pont du Château, France). Detailed demographic, clinical, and biological data were obtained during the inclusion visit using an electronic case-report form. Follow-up included systematic visits annually and spontaneous reports for particular events, which were recorded on specific data forms.7
This study was observational, and decisions about treatment combination, treatment timing, and screening for the progression of fibrosis were made by the clinician, according to French national recommendations, based on EASL guidelines.10
The stages of fibrosis were assessed by the FibroTest™ (F0–F4) and activity grades (A0–A3) by the ActiTest™ according to the manufacturer’s instructions using the equivalence with the histological METAVIR scoring system, and standard validated cut-offs and reliability criteria.[14], [15], [16], [17] The FibroTest™ is approved by European guidelines and by the French national healthcare system for the surveillance of CHC. Several expert centres routinely perform these tests. For the remaining patients, FibroTest™ was measured on the available centralised biobank, independently from patient characteristics.
Outcomes
The co-primary study outcome was the NPV of LCR1-LCR2 at 5 years for the occurrence of HCC as well as survival without HCC according to the predetermined LCR1-LCR2 cut-off, adjusted for HCC risk variables and for the response to HCV treatment, quantified using time-dependent Cox proportional hazards models. This core analysis used the algorithm assuming that only patients with cirrhosis (FibroTest™ >0.74), and patients without cirrhosis (FibroTest™ 0.74) and high LCR1-LCR2 would require screening. The binary result of the LCR1-LCR2 algorithm, the risk of HCC, was identified here as either a ‘low’ or ‘high’ risk. This study was registered with ClinicalTrials.gov (NCT01953458). Data for incident HCC included the number of tumours at diagnosis, the largest nodule size, total size, and diagnostic imaging procedures.
The secondary outcome was the prognostic performance of LCR1-LCR2, using the same endpoints but adding patients with severe fibrosis (METAVIR stage F3) defined as numerous septa.14 This analysis used the algorithm assuming that patients with cirrhosis or severe fibrosis (FibroTest™ >0.58), and high LCR1-LCR2 should be screened. This surveillance strategy, including both stages F3 and F4, is recommended by the EASL10 and the American Gastroenterological Association in patients before and after DAA with a SVR,9 as well as in other published reviews.1 However, LCR1-LCR2 does not differentiate between F4 and F3. Thus, we hypothesised that this strategy would decrease the need for the AFP assessment as a result of the high NPV of LCR1 in the first step, regardless of the fibrosis stage.
Six post hoc analyses of test performance were performed. First, LCR1-LCR2 was compared with standard surveillance (the reference) in patients with cirrhosis only and using AFP at the standard 20 ng/ml cut-off.2 Second, LCR1-LCR2 performance was assessed in patients aged 50 years or older, which could improve the cost-effectiveness, as suggested in the Original Study.11 These results were compared with standard surveillance in the same age subset. Then, to evaluate the sustainability of the NPV over time, we analysed the results at 10 years and during the entire follow-up. We also assessed the impact of using a 90-day instead of a 1-year exclusion period to prevent an ‘immortal person-time’ bias.18 The exclusion of HCC diagnosed during the first year and of a follow-up of less than 1 year was justified in the Original Study to reduce the bias of the presence of contemporaneous HCC when performing LCR1-LCR2. The fifth analysis evaluated the subset of patients with and without SVR. The sixth analysis compared the risk of HCC according to LCR1-LCR2 results to the risk of HCC in the general population obtained from national cancer registries, with standardisation for age and sex.19
In a final pooled analysis, we combined the present external validation database with the updated Original Study to check the overall NPV of LCR1-LCR2 with the best power.
Statistical analysis
A post hoc calculation was based on the results of the Original Study of LCR1-LCR2 in CHC,11 which identified 45 cases of HCC in the 3,390 patients without cirrhosis, and 88 in the 1,347 patients with cirrhosis. More than 100 events were considered to be an appropriate sample size for external validation of a multivariate prognostic model.20 These results suggested that at least 3,000 patients without cirrhosis and with CHC, and 800 with cirrhosis were necessary for external validation of the model for use in the same context (expert centres in France).
Survival time was calculated as the time between the first assessment of LCR1-LCR2 and the date of the primary outcome, the last follow-up visit, the date of death, or July 15, 2020, whichever occurred first. HCCs that occurred within the first year or after less than 1 year of follow-up were excluded from the primary analysis.
We used a multivariate Cox proportional hazards model with exposure to HCV treatment modelled as a time-varying covariate in our main analysis. This analysis was adjusted for the baseline values of all predictor variables previously identified as associated with the occurrence of HCC: age, sex, geographical origin (France vs. other), HCV genotype 3, past excessive alcohol use, arterial hypertension, diabetes, and response to HCV treatment. The categorisation of continuous covariates was based on clinically relevant thresholds determined a priori (all biological variables) or quartile limits (age and time since HCV diagnosis). To prevent an incorporation bias, the FibroTest™, which defines the stages of fibrosis, was not included in the HCC risk covariables. All analyses were performed in duplicate, and the final decision was made by 2 authors (F.C. and S.P.), independently from the LCR1-LCR2 inventor team. LCR1-LCR2 values were assessed at the first available date. Other characteristics were measured at inclusion in the Hepather cohort.7
To better characterise the potential effect of a SVR in patients exposed to antivirals compared with untreated patients, the exposure period was divided into the pretreatment period (from the start of follow-up to 3 months after the last day of treatment in treated patients or the end of follow-up in untreated patients), and the period with a measurable SVR status (from 3 months after the last day of DAA treatment to the end of follow-up), which were regarded as time-dependent covariates in the Cox model.7 SVR status was assessed after the first DAA treatment and was updated if a patient received several consecutive treatments after the first analysis.7 Given that the goal of this study was to validate a predictive test for the incidence of HCC, only HCCs that occurred at least 1 year after the LCR1-LCR2 assessment were considered to be an incident HCC in the Original Study.11
The same methods were used for the post hoc analyses as those used for the main endpoints. These methods were applied to the subset of patients aged 50 years or older, at 10 years, and at the end of follow-up, as well as using an exclusion period of 90 days instead of 1 year, and in patients with or without SVR. We did not perform a cost-effectiveness analysis but did assess the number of patients needed to screen (NNS) to identify 1 HCC. We estimated the standardised incidence ratio (SIR) to assess whether the risk of HCC in patients with a low LCR1-LCR2 was similar to that in the general population.19,21 SIR results were compared with the Chi-squared test.
Baseline characteristics were compared using the Mann–Whitney test for quantitative variables or the Fisher’s exact test for categorical variables. Kaplan–Meier curves were drawn to compare survival without HCC. Incidence and 95% CIs were estimated with an exact method based on the Poisson distribution. For the pooled analysis of the updated Original Study cohort, which started in 1997, the SVR was not limited to DAA treatments. Analyses were performed in duplicate, blind to LCR1-LCR2 values, with SAS version 9.4. NCSS-12.0 and R software, including timeROC library.
Results
Flow of participants through the study
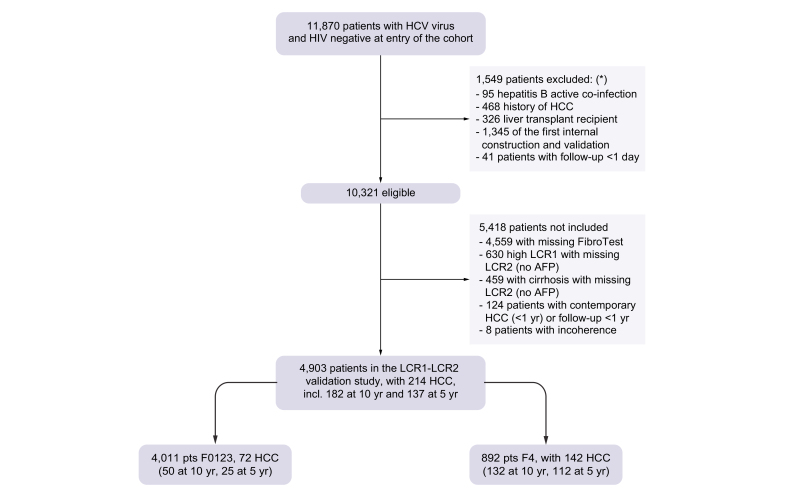
Between August 6, 2012, and December 31, 2015, 11,870 anti-HCV-positive patients with CHC infection at entry were recruited to the ANRSCO22 Hepather cohort. A total of 10,321 were eligible for the core study on treatment impact and 4,903 remained for LCR1-LCR2 external validation (Fig. 1).7 The characteristics of the included patients are presented in Table 1. A total of 5,418 patients were not included in the present diagnostic study mainly because of missing FibroTests™ (n = 4,559) or missing LCR2 (n = 1,106). Compared with not-included patients, excluded patients had more risk factors associated with HCC, including age, cirrhosis stage, genotype 3, past excessive alcohol use, type 2 diabetes mellitus, and arterial hypertension, but the geographical origins were similar (Table S1).
Fig. 1.
Flow chart of participants in the study.
Patients could have more than 1 reason for exclusion. AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk.
Table 1.
Characteristics of Hepather patients included in the external validation of LCR1-LCR2 algorithm.
| Characteristic | Hepather cohort included (n = 4,903) | Missing data |
|---|---|---|
| HCC, n (%) | 214 (4.4) | 0 |
| LCR1-LCR2 algorithm, n (%) | 0 | |
| Low risk | 3,755 (76.6) | |
| High risk | 1,148 (23.4) | |
| Follow-up time, years, median (IQR) | 5.8 (4.2–11.4) | 0 |
| Age at inclusion, years (IQR) | 55.6 (49.0–64.4) | 0 |
| Age at FibroTest™ time, years (IQR) | 52.6 (45.1–61.1) | 0 |
| Men, n (%) | 2,412 (49.2) | 0 |
| Body mass index (kg/m2) | 24.4 (21.9–27.4) | 18 |
| Smoker, n (%) | 4 | |
| At inclusion | 1,689 (34.5) | |
| Previously | 2,985 (61.0) | |
| Geographical origin, n (%) | 39 | |
| France or Eastern Europe | 3,488 (71.7) | |
| Asia | 108 (2.2) | |
| North Africa | 427 (8.8) | |
| Other (mostly Sub-Saharan) | 841 (17.3) | |
| Past excessive alcohol use, n (%) | 1,225 (25.0) | 0 |
| Time since HCV infection, (IQR) | 10.1 (3.3–16.6) | 88 |
| HCV contamination cause, n (%) | 1,598 | |
| Drug usage | 836 (25.3) | |
| Transfusion | 1,094 (33.1) | |
| Other or unknown | 1,375 (41.6) | |
| HCV genotype, n (%) | 24 | |
| 1 | 3,300 (67.6) | |
| 2 | 332 (6.8) | |
| 3 | 547 (11.2) | |
| 4 | 583 (11.9) | |
| 5–7 | 117 (2.4) | |
| Fibrosis at inclusion using Hepather criteria, n (%) | 210 | |
| F0 | 1,271 (27.1) | |
| F1 | 1,055 (22.5) | |
| F2 | 540 (11.5) | |
| F3 | 801 (17.1) | |
| F4 | 1,026 (21.9) | |
| Fibrosis at first FibroTest™ assessment, n (%) | 0 | |
| F0 (≤0.21) | 1,605 (32.7) | |
| F1 (>0.21) | 1,098 (22.4) | |
| F2 (0.48) | 472 (9.6) | |
| F3 (0.58) | 836 (17.1) | |
| F4 (0.74) | 892 (18.2) | |
| Alanine aminotransferase (IU/L) at inclusion (IQR) | 55 (36–90) | 58 |
| Type 2 diabetes mellitus, n (%) | 506 (10.3) | 0 |
| Arterial hypertension, n (%) | 1,313 (26.8) | 1 |
| Alpha-fetoprotein class (ng/ml), n (%) | 728 | |
| <6 | 2,841 (68.0) | |
| 6 to <10 | 654 (15.7) | |
| 10 to <20 | 420 (10.1) | |
| 20 to <120 | 236 (5.6) | |
| >120 | 24 (0.6) | |
| HCV treatment, n (%) | 396 | |
| With sustained virological response | 3,405 (75.5) | |
| Without sustained virological response | 1,102 (24.5) |
HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk.
Primary outcomes
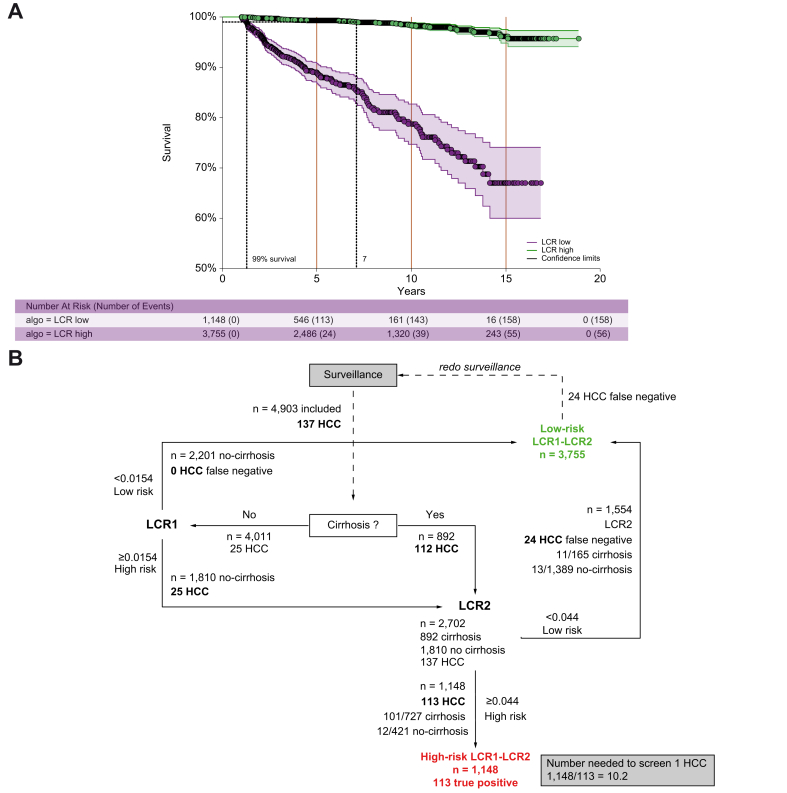
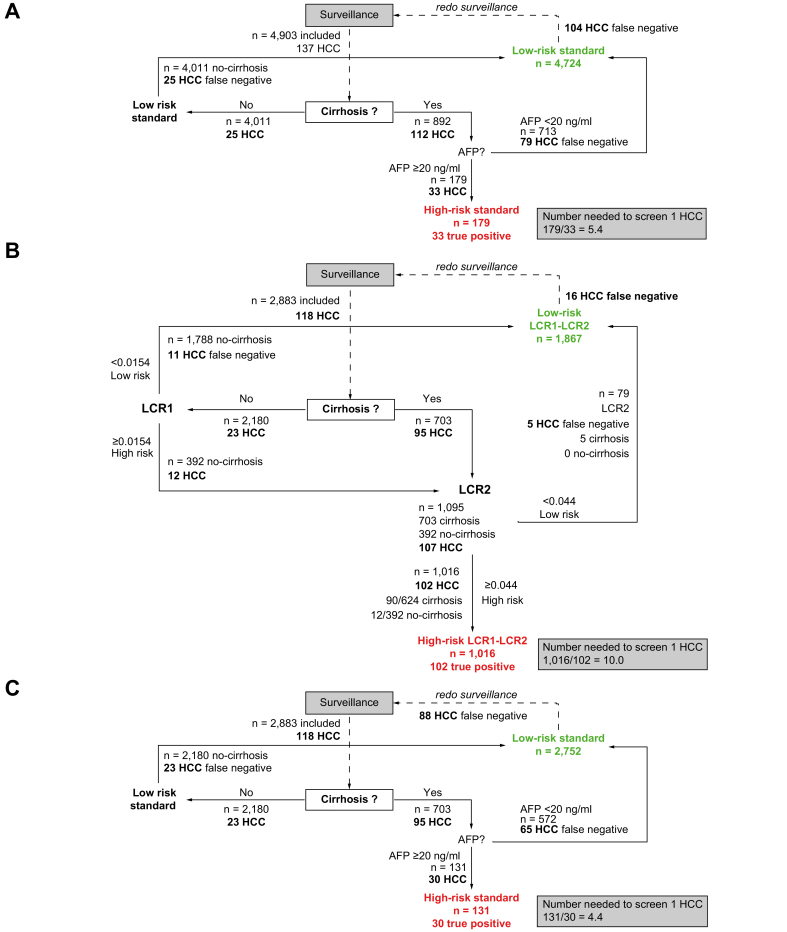
A total of 4,903 patients, 892 (18.2%) with baseline cirrhosis, were included in the study. Seventy-seven percent of patients had a SVR for a median of 5.7 (IQR 4.2–11.3) years, and 214 occurred at the end of follow-up. Only 24 cases of HCC occurred at 5 years in the 3,755 patients with low-risk LCR1-LCR2, with a prevalence of 76.6% (95% CI 75.4–77.8) and a NPV of 99.4% (95% CI 99.1–99.6) compared with 113 HCCs in 1157 patients with high-risk LCR1-LCR2 (Fig. 2A). The diagnostic performances are detailed in Table 2. The false negative rate was 17.5% (24/137; 95% CI 11.6–24.9) and the NNS was 10.2 (Fig. 2B).
Fig. 2.
Survival without HCC according to LCR1-LCR2 cut-offs: main outcomes.
(A) Survival without HCC. (B) Number of patients needed to screen 1 HCC. HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk.
Table 2.
Performance summary for LCR1-LCR2 algorithm and standard surveillance according to surveillance duration.
| HCC incidence | |||
|---|---|---|---|
| Surveillance option | Cases |
HCC incidence |
|
| HCC |
Standardised ratio incidence |
||
| n | n | Low risk/high risk (95% CI) | |
| 5-years follow-up, 1-year HCC exclusion | |||
| Primary outcome 5 years LCR1-LCR2 | 4,903 | 137 | 9.80 (6.3–14.6)/56.5 (46.6–67.9) |
| Secondary outcome F3–F4 LCR1-LCR2 | 4,903 | 137 | 9.80 (6.3–14.6)/56.5 (46.6–67.9) |
| Post hoc analyses | |||
| Standard, cirrhosis only | 4,903 | 137 | 24.5 (20.0–29.7)/165.0 (113.6–231.7) |
| 50 years or older, LCR1-LCR2 | 2,883 | 118 | 7.1 (4.1–11.6)/51.0 (41.6–61.9) |
| 50 years or older, cirrhosis only | 703 | 95 | 2.2 (0.7–5.2)/45.0 (36.2–55.3) |
| Other follow-ups | |||
| 10 years’ follow-up, LCR1-LCR2 | 4,903 | 182 | 9.8 (6.9–13.3)/39.7 (33.5–46.8) |
| Maximum follow-up, LCR1-LCR2 | 4,903 | 214 | 8.1 (6.1–10.5)/49.4 (42.0–57.7) |
| 5-years’ follow-up, 90 days HCC exclusion | 4,978 | 181 | 12.2 (8.2–17.4)/75.5 (63.9–88.6) |
| Performance | ||||
|---|---|---|---|---|
| Follow-up | LCR1-LCR2 and standard surveillance performance |
|||
| Negative predictive value (%) (95% CI) | Positive predictive value (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
| Primary outcome 5 years | 99.4 (99.2–99.6) | 9.8 (9.0–10.6) | 82.5 (81.4–83.6) | 78.3 (77.1–79.5) |
| Secondary outcome F3–F4 | 99.4 (99.2–99.6) | 9.8 (9.0–10.6) | 82.5 (81.4–83.6) | 78.3 (77.1-79.5) |
| Post hoc analyses | ||||
| Standard, cirrhosis only | 97.8 (97.6–98.0) | 19.0 (17.9–20.1) | 24.1(22.9–25.3) | 97.0 (96.5–97.5) |
| 50 years or older | 99.1 (98.7–99.5) | 10.0 (8.9–11.1) | 85.1 (81.4–87.7) | 66.9 (65.2–68.6) |
| 50 years or older with cirrhosis | 99.1 (98.7–99.5) | 10.0 (8.9–11.1) | 85.1 (81.4–87.7) | 66.9 (65.2–68.6) |
| 10 years’ follow-up | 99.0 (98.8–99.2) | 12.5 (11.6–13.4) | 78.6 (77.5–79.8) | 78.7 (77.5–-79.9) |
| Maximum follow-up | 98.5 (98.2–98.8) | 13.8 (12.8–14.8) | 73.8 (72.6–75.0) | 78.9 (77.8–80.0) |
| 5 years’ HCC exclusion (90 days) | 99.2 (99.0–99.4) | 12.6 (12.8–14.8) | 83.4 (82.5–84.3) | 78.3 (77.2–79.4) |
HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk.
After adjustment for exposure to antivirals, age, sex, geographical origin, HCV genotype 3, alcohol consumption, type 2 diabetes mellitus, and arterial hypertension, the multivariable LCR1-LCR2 Cox hazard ratio (primary endpoint) was still highly significant (10.8; 95% CI 8.1–14.3). The unadjusted univariate hazard ratio for high vs. low-risk LCR1-LCR2 was slightly higher (13.9; 95% CI 10.2–19.0; p <0.001) (Table 3).
Table 3.
Factors associated with survival without HCC (uni- and multivariate analyses).
| Factor | Time-dependent hazard ratio |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
|||||
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| LCR1-LCR2 algorithm | 13.94 | 10.22–19.01 | <0.001 | 10.79 | 8.14–14.31 | <0.001 |
| Sex (men vs. women) | 2.68 | 2.00–3.59 | <0.001 | 2.26 | 1.75–2.92 | <0.001 |
| Age (years) at FibroTest™ time | 1.06 | 1.04–1.07 | <0.001 | |||
| ≤40 (reference) | ||||||
| 40–60 | 4.84 | 2.13–10.98 | <0.001 | 1.61 | 0.83–3.11 | 0.1573 |
| >60 | 9.03 | 3.95–20.65 | <0.001 | 1.40 | 0.70–2.76 | 0.3478 |
| Geographical origin (European vs. other) | 1.41 | 0.97–2.04 | 0.0711 | 1.59 | 1.19–2.11 | 0.0016 |
| Past excessive alcohol use (yes vs. no) | 1.87 | 1.41–2.48 | <0.001 | 1.00 | 0.79–1.27 | 0.9951 |
| Ever smoked (yes vs. no) | 1.29 | 0.97–1.71 | 0.0797 | 1.03 | 0.79–1.35 | 0.8171 |
| Treatment-naive vs. treated | 0.31 | 0.22–0.44 | <0.001 | 0.54 | 0.43–0.68 | <0.001 |
| HCV genotype 3 (other reference) | 2.86 | 2.09–3.95 | <0.001 | 2.94 | 2.29–3.77 | <0.001 |
| Diabetes (yes vs. no) | 2.73 | 1.98–3.77 | <0.001 | 1.30 | 1.01–1.68 | 0.0396 |
| Arterial hypertension (yes vs. no) | 1.78 | 1.35–2.34 | <0.001 | 1.44 | 1.15–1.81 | 0.0018 |
| Response to DAA | ||||||
| Unexposed | 1.00 | |||||
| SVR | 1.00 | 0.79–1.29 | <0.001 | 0.41 | 0.31–0.53 | <0.001 |
| Non-responder | 4.06 | 2.70–6.10 | 1.67 | 1.10–2.53 | ||
| Unknown | 1.10 | 0.59–2.03 | 0.61 | 0.33–1.14 | ||
DAA, direct-acting antivirals; HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk; SVR, sustained virological response.
The characteristics of incident HCCs according to low- or high-risk LCR1-LCR2 cut-offs are reported in Table S2A. Most incident HCCs were potentially curable and all were smaller than 30 mm. Nodular macroscopic patterns were identified in 91.7% of patients with low LCR1-LCR2 and 88.6% in those with high LCR1-LCR2. The 24 patients with HCC and a low LCR1-LCR2 had a higher prevalence of fibrosis stage F1 and F2, and HCV genotype 3; 70% were men; 21% had type 2 diabetes mellitus; and had a greater frequency of AFP between 6 and 20 ng/ml compared with patients without HCC and a low LCR1-LCR2 (Table S2B). Other comparisons of the characteristics between low- and high-risk patients are presented in Table S3.
Secondary outcomes
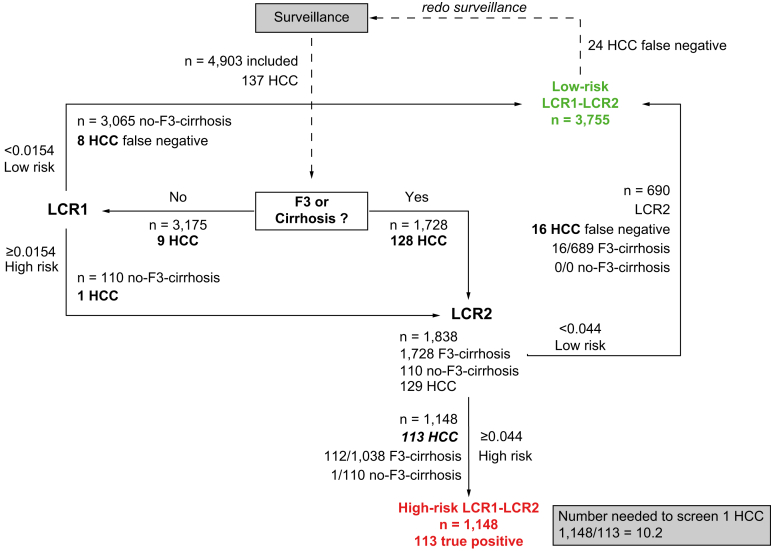
A total of 1,728 patients out of 4,903 (35.7%) had severe fibrosis or cirrhosis (F3-F4) (Table 1). Sixteen cases of HCC occurred in patients with F3 at 5 years (Fig. 3) compared to 112 in patients with cirrhosis (Fig. 2B). Assessing LCR2 in F3 and cirrhosis rather than cirrhosis alone decreased the NNS of LCR2 by 32.0% from 2,702 to 1,838.
Fig. 3.
Secondary outcome of survival without HCC in patients with surveillance of both severe fibrosis (F3) and cirrhosis (F4).
Relative number of LCR1 and LCR2 assessments and number of patients needed to screen 1 HCC. Compared with the ‘cirrhosis-only’ option, assessing LCR2 in F3 and cirrhosis decreased the NNS of LCR2 by 32.0% from 2,702 when cirrhosis-only was the first step to 1,838. HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk; NNS, number needed to screen.
Six post hoc analyses
Comparison of LCR1-LCR2 with standard surveillance in patients with cirrhosis only and using AFP at the standard 20 ng/ml cut-off
The false negative rate with standard surveillance in the population with cirrhosis alone was 3 times higher than with LCR1-LCR2 (Fig. 2B) at 75.9% (104/137; 95% CI 67.9–62.8) (Table 2), and the NNS was 2 times lower, at 5.4 (Fig. 4A).
Fig. 4.
Standard surveillance and LCR1-LCR2 post hoc analyses.
(A) Standard surveillance in cirrhosis only. Number of patients needed to screen was reduced and false negative increased compared to LCR1-LCR2. (B) LCR1-LCR2 in patients 50 years or older. Number of patients needed to screen 1 HCC. There was no significant difference in the number of patients needed to screen and the false negative rate compared to LCR1-LCR2. (C) Standard surveillance in patients 50 years or older: number of patients needed to screen 1 HCC. The number of patients needed to screen was reduced and false negative increased compared to LCR1-LCR2 in the same age subset. HCC, hepatocellular carcinoma; LCR, Liver Cancer Risk.
Assessment of LCR1-LCR2 performance in patients aged 50 years or older
There was no significant difference in the false negative rate with LCR1-LCR2 in patients aged 50 years or older compared with the overall population (Fig. 2B) at 13.6% (16/118; 95% CI 67.9–62.8) or in the NNS (10.0) (Fig. 4B and Table 2). Compared with standard surveillance in the same age subset (Fig. 4C and Table 2), there was a persistently lower significant false negative rate and a lower NNS withLCR1-LCR2.
Sustainability of LCR1-LCR2 over time
In the 3,755 patients with low LCR1-LCR2, univariate survival without HCC at 5 years was 99.4% (95% CI 99.1–99.6), 99.0% (95% CI 98.6–99.3) after 10 years of follow-up, and 98.5% (95% CI 98.2–98.8) at 20 years (Fig. 2 and Table 2).
Impact of using a 90-day instead of a 1-year exclusion period
The performance of LCR1-LCR2 at 5 years was similar when only patients with less than 90 days of follow-up were excluded. Only 30 HCCs occurred at 5 years in the 3,784 patients with low LCR1-LCR2 compared with 151 out of 1,194 with high LCR1-LCR2, for a NPV of 99.2% (95% CI 98.9–99.5). The positive predictive value was 12.6% (95% CI 10.8–14.7). The diagnostic performances are detailed in Table 2.
Comparison of the subset of patients with and without SVR
The characteristics of the 3,405 patients with a SVR (Table S4A) and the 1,102 patients without a SVR (Table S4B) were similar to those of the core population (Table 1). The same high NPV (99%) for the occurrence of HCC at 5 years was identified in patients with (Fig. S1A) or without a SVR (Fig. S1B).
Comparison of the HCC standardised risk ratio in the low LCR1-LCR2 subset vs. the risk observed in the general population
The incidence of HCC standardised by sex and age in patients with a low LCR1-LCR2 was significantly higher than the incidence assessed in the French general population (SIR = 9.8; 95% CI 6.3–14.6; p <0.001; Table S5A). Patients with a high-risk LCR1-LCR2 still had a 5 times higher SIR (56.8; 95% CI 46.8–68.3) compared with patients with a low risk LCR1-LCR2 (Table S5B).
Updated results of the original GHPS cohort and pooled analysis
The results of a subset of 1,509 patients with HCV from the Original Study11 were updated in October 2020. The patient characteristics are set out in Table S6. Three HCCs occurred at 5 years in 656 patients with a low LCR1-LCR, compared with 46 HCC in 837 patients with a high-risk LCR1-LCR2, for a survival of 99.7% (95% CI 99.2–1.00) and 96.0% (95% CI 94.6–97.4; p <0.001), without HCC, respectively (Table S7). These performances were similar to those of the present external validation (Fig. 2).
A total of 6,412 pooled patients (4,903 + 1,509 = 6,412) were analysed. Patient characteristics are detailed in Table S8. Survival without HCC (Fig. S2) at 20 years was 96.2% (95% CI 94.9–97.4).
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and the Standards for the Reporting of Diagnostic Accuracy Studies (STARD) checklists are reported in Tables S9 and S10, respectively.
Discussion
This multicentre external cohort of patients with CHC validates the performance of LCR1-LCR2 to assess the risk of HCC at 5 years. The NPV was 99.4% (95% CI 99.1–99.6), which is similar to the 99.5% (95% CI 99.0–99.7) previously observed in the Original Study.11 This algorithm could help clinicians reassure 76% of patients with CHC with low-risk LCR1-LCR2 scores and discuss surveillance in those with high-risk LCR1-LCR2 scores. However, this study has certain strengths and limitations.
Strengths
Several limitations of the Original Study were corrected in the present study, in particular the absence of external validation, the sample size, the multiple causes of chronic liver diseases, the limited number of risk factors,11 and the absence of time-dependent multivariate analysis to take into account the impact of SVR on survival without HCC.7 Furthermore, according to the STROBE and STARD checklists, we also assessed the long-term performances at 10 years and at the end of follow-up, and used a 90-day HCC exclusion to prevent an ‘immortal person-time’ bias.18
Sample size and range of patients
Given the large prospective Hepather cohort, our study had sufficient power to assess the performance of LCR1-LCR2 at 5 years and to analyse the robustness of the results with 6 post hoc analyses. The power of the study was appropriate for this external validation at 5 years with 146 HCC events, which is more than the recommended 100 events.21 It was also possible to check the consistency of the NPV after up to 15 years of follow-up, with 1,973 and 1,481 patients still at risk at 7 and 10 years, respectively. With the very early use of the FibroTest™ in the French population, we also had a validated method to assess the stage of fibrosis at inclusion in these patients. This provided a large range of patients, including 49.6% without or with minimal fibrosis, as well as 24.5% without SVR. Indeed, even in the DAA era, 72.9% of HCV-associated HCC is predicted to develop in patients without SVR in the USA between 2012 and 2040.22 Our ambispective analysis was useful to assess health outcomes with a long induction period and exposure.12
Identification of patients with HCV with a very low risk of HCC
The remaining incidence of HCC in patients with CHC with and without cirrhosis despite the effectiveness of DAA is a major issue.[1], [2], [3], [4], [5],7,9 The most recent best-practice advice states that ‘patients with severe liver fibrosis (F3) or cirrhosis (F4) at the time of treatment represent the highest-risk group for HCC after a treatment-induced sustained virological response. These patients should stay in HCC surveillance’.3 The present study validated LCR1-LCR2 in 2 screening process steps in patients with and without cirrhosis, first, as a blood-based risk stratification biomarker, and second, in terms of validation of its screening in a large cohort with CHC.
A robust blood test for mixed chronic liver diseases
In the results of the Original Study, the NPVs of LCR1-LCR2 were similar in patients with alcoholic liver disease and nonalcoholic fatty liver disease, and in those with HCV.11 This is an advantage because of the long period of possible exposure to these risks in patients with HCV before or after SVR. This also supports the generalisation of the results of the present study to patients with mixed chronic liver diseases.
A sensitive test that could be updated with specific markers of HCC other than AFP
LCR1-LCR2 was constructed to be highly sensitive with the combined components and highly specific with AFP. Thus, the test could be updated to improve performance with other combinations and specific HCC markers, such as AFP-L3, decarboxy-prothrombin, or glycans.23
Limitations
The assessment of relative risk and high NPV is not enough to confirm the efficacy of this test as an updated screening tool. The present study externally validated LCR1-LCR2 as a first step, showing that 76% of the population of interest could be reassured. The very high NPV of our results suggests that, for at least 5 years, patients with low LCR1-LCR2 do not require surveillance every 6 months by ultrasound with or without AFP.2 The sustainability of NPV was excellent and was still 99% at 10 years (Fig. 2). Thus, LCR1-LCR2 could be used to assess the risk of the development of HCC in patients with CHC.
We acknowledge that our population mainly (72%) included patients from Europe; however, 26% were of African origin, and there was a wide spectrum of HCV genotypes. These results must be confirmed in more patients from Asia. The 24 false negative patients who developed HCC despite the low LCR1-LCR2 were still significantly different from the true negative cases, including for male sex, HCV genotype 3, and type 2 diabetes mellitus (Table S2B). This profile suggests the presence of associated HCC risk factors, metabolic factors, or pre-existing liver cell adenoma in these patients.24
We also acknowledge that the present study was designed as an external validation of LCR1-LCR2, and not as a prospective comparison with other prognostic biomarkers, such as transient elastography or Fibrosis-4 (FIB-4).25 Given the occurrence of sampling errors, biopsy itself has 30% false results compared with a true gold standard test.26,27 Therefore, 15% of F3 are F2, and vice versa. Thus, all NITS cut-offs [such as FibroTest™ or vibration-controlled transient elastography (VCTE)] might have false F0–F2 vs. F3–F4 diagnoses. This is another reason to validate the HCC risk calculator in all patients with CHC from F0 to F4. In CHC, an intention-to-diagnose and face-to-face comparison showed that the reliability of the FibroTest™ outperformed VCTE and that its area under the receiver operating curve (AUROC) was higher than that of FIB4.25
LCR1-LCR2 was constructed to have a high sensitivity based on the combined components and significant specificity because of AFP. Although the LCR1-LCR2 score uses the same components (except bilirubin) as the FibroTest™, the weights of the components in the algorithm steps are different. Thus, the algorithms used in LCR1-LCR2 can be adapted to new HCC-specific markers, such as AFP-L3, decarboxy-prothrombin, or glycans.23
Also, the incidence of HCC standardized by gender and age in the patients with low-risk LCR1-LCR2 is still significantly higher, than the incidence in the French general population, SIR = 9.8 (95% CI 6.3–14.6; p <0.001). There is no clear explanation for this difference, either because of an underestimation of the incidence of HCC in the general population or an overestimation in patients with CHC. In addition, the incidence of HCC standardised by sex and age in the patients with low-risk LCR1-LCR2 was significantly higher than the incidence in the general French population (56.8; 95% CI 46.8–68.3). One explanation could be the recent increase in ultrasonography and AFP tests in patients with CHC. In the short term, screening increases the incidence, and advances both the year and age at diagnosis, whereas, in the long term, it reduces the incidence through early detection.
We also did not assess the cost-effectiveness of LCR1-LCR2. However, this algorithm can be assessed with the components of the FibroTest™, which is already recommended for surveillance in patients with CHC regardless of disease stage, and AFP is only required in 24% of patients, together with ultrasound. Even if all patients are treated by DAA, the stage of fibrosis must still be assessed.3 Therefore, a single blood test assessing both the stage of fibrosis by the validated, sensitive, FibroTest™ and the risk of HCC should help both the patient and the clinician. This could reduce the number of patients requiring repeated imaging.
Finally, we agree with others that stopping the surveillance of low-risk groups is questionable, and that the intensification of screening programs in intermediate- or high-risk groups is a challenge that would improve compliance with surveillance recommendations.[3], [4], [5],10,[28], [29], [30]
Conclusions
In conclusion, LCR1-LCR2 is a robust blood test for use in the assessment of the risk of developing HCC in patients with CHC.
Financial support
The GHPS cohort is supported by the HCC Multi-technological consortium (HECAM C1410019W). The Hepather cohort is supported by INSERM-ANRS (France Recherche Nord & Sud Sida-HIV Hépatites), ANR (Agence Nationale de la Recherche), DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. The funder contributed to the study design and the writing of the report. The funder had no role in data collection. Data analysis or data interpretation was performed in duplicate both by the founders and non-founders. The corresponding author had full access to all data in the study and F.C. and S.P. had final responsibility for the decision to submit for publication.
Authors’ contributions
Experiment conception and design: TP. Performed the experiments: TP, VP, VdL, FZ, DS, DT, HF, OD, PM, VP, VR, SP. Data analysis: TP, OD, JML, FC. Drafting the manuscript: TP, CH, JML, SP, FC. Approval of the final version of the manuscript: all authors.
Data availability statement
TP is acting as the submission's guarantor. Data from this study are available, upon request, from F.C. (fabrice.carrat@iplesp.upmc.fr).
Conflicts of interest
T.P. is the inventor of FibroTest™, LCR1, and LCR2, and founder of BioPredictive,; the patents belong to Assistance Publique-Hôpitaux de Paris. V.P. and O.D. are full-time employees of BioPredictive. The remaining authors have declared no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100298.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F., Singal A.G. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157:54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal A.G., Lok A.S., Feng Z., Kanwal F., Parikh N.D. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol. 2020:S1542–S3565. doi: 10.1016/j.cgh.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain M.K., Rich N.E., Ahn C., Turner B.J., Sanders J.M., Adamson B. Evaluation of a multifaceted intervention to reduce health disparities in hepatitis C screening: a pre-post analysis. Hepatology. 2019;70:40–50. doi: 10.1002/hep.30638. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T., Deckmyn O., Munteanu M., Ngo Y., Drane F., Castille J.M. Awareness of the severity of liver disease re-examined using software-combined biomarkers of liver fibrosis and necroinflammatory activity. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrat F., Fontaine H., Dorival C., Simony M., Diallo A., Hezode C. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T., Moussalli J., Munteanu M., Thabut D., Lebray P., Rudler M. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59:675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Singal A.G., Lim J.K., Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156:2149–2157. doi: 10.1053/j.gastro.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T., Peta V., Deckmyn O., Munteanu M., Moussalli J., Ngo Y. LCR1 and LCR2, two multi-analyte blood tests to assess liver cancer risk in patients without or with cirrhosis. Aliment Pharmacol Ther. 2019;49:308–320. doi: 10.1111/apt.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peta V., Ziol M., Imbert-Bismut F., Sutton A., Monneret D., Munteanu M. External validation of an algorithm combining multi-analyte blood tests (FibroTest-LCR1-LCR2) to identify subjects at risk of in patients with chronic liver disease. GastroHep. 2019;1:146–153. [Google Scholar]
- 13.EFSA Scientific Committee. More S., Bambidis V., Benford D., Bragard C., Hernandez-Jerez A. Draft for internal testing Scientific Committee guidance on appraising and integrating evidence from epidemiological studies for use in EFSA's scientific assessments. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T., Munteanu M., Deckmyn O., Ngo Y., Drane F., Messous D. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol. 2011;11:39. doi: 10.1186/1471-230X-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poynard T., Munteanu M., Deckmyn O., Ngo Y., Drane F., Castille J.M. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: proof of concept and first application in a large population. J Hepatol. 2012;57:541–548. doi: 10.1016/j.jhep.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Imbert-Bismut F., Ratziu V., Pieroni L., Charlotte F., Benhamou Y., Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 18.Mi X., Hammill B.G., Curtis L.H., Lai E.C., Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35:4824–4836. doi: 10.1002/sim.7019. [DOI] [PubMed] [Google Scholar]
- 19.Defossez G., Le Guyader-Peyrou S., Uhry Z., Grosclaude P., Colonna M., Dantony E. Saint-Maurice: Sante publique France; 2019. Estimations nationales de l’incidence et de la mortalite par cancer en France metropolitaine entre 1990 et 2018. Volume 1 – Tumeurs solides. Étude à partir des registres des cancers du réseau Francim. [Google Scholar]
- 20.Collins S.G., Ogundimu E.O., Altman D.G. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. 2016;35:214-26. doi: 10.1002/sim.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslow N.E., Day N.E. IARC; Lyon: 1987. Statistical Methods in Cancer Research Volume II: the design and analysis of cohort studies. [PubMed] [Google Scholar]
- 22.Chen Q., Ayer T., Adee M.G., Wang X., Kanwal F., Chhatwal J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis C in the era of direct-acting antiviral agents. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peta V., Zhu J., Lubman D.M., Huguet S., Imbert-Bismut F., Bolbach G. Input of serum haptoglobin fucosylation profile in the diagnosis of hepatocellular carcinoma in patients with non-cirrhotic liver disease. Clin Res Hepatol Gastroenterol. 2020;44:681–691. doi: 10.1016/j.clinre.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradis V., Zalinski S., Chelbi E., Guedj N., Degos F., Vilgrain V. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 25.Houot M., Ngo Y., Munteanu M., Marque S., Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther. 2016;43:16–29. doi: 10.1111/apt.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedossa P., Dargère D., Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 27.McHugh L.C., Snyder K., Yager T.D. The effect of uncertainty in patient classification on diagnostic performance estimations. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costentin C.E., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead-time adjusted survival of patients with compensated viral cirrhosis: a multi-center cohort study. Gastroenterology. 2018;155:431–442. doi: 10.1053/j.gastro.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Audureau E., Carrat F., Layese R., Cagnot C., Asselah T., Guyader D. Personalized surveillance for hepatocellular carcinoma in cirrhosis - using machine learning adapted to HCV status. J Hepatol. 2020;73:1434–1445. doi: 10.1016/j.jhep.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 30.Ioannou G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2020;74:458–465. doi: 10.1016/j.jhep.2020.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TP is acting as the submission's guarantor. Data from this study are available, upon request, from F.C. (fabrice.carrat@iplesp.upmc.fr).