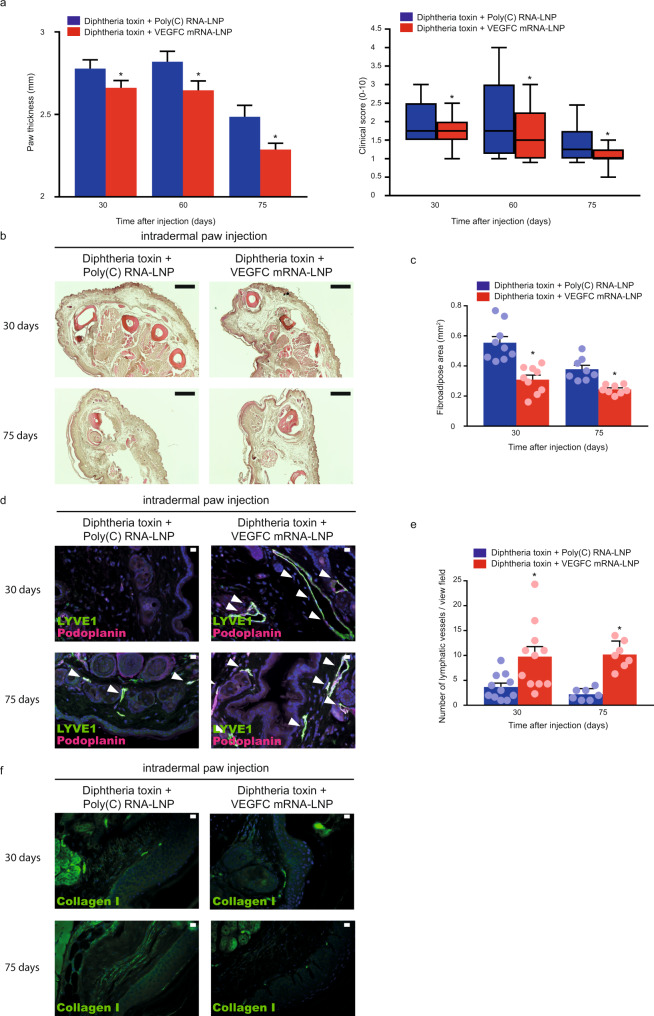
Fig. 9. VEGFC mRNA-LNP treatment reverses experimental lymphedema.
a Monitoring paw thickness and clinical score in Diphtheria toxin and tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice intradermally injected with 1 µg of Poly(C) or VEGFC mRNA-LNPs. Quantitative data are represented as mean and SEM for paw thickness and box shows median and 25th to 75th percentiles and whiskers show 10th–90th percentiles for clinical score in 15–31 mouse paws in each group (two-tailed, paired T-test, for paw thickness P = 0.0090 on days 30 for 31 mice, P = 0.0053 on day 60 for 25 mice and P = 0.0082 on day 75 for 15 mice. Two-tailed Wilcoxon signed-rank test for clinical score P = 0.0050 on day 30 for 31 mice, P = 0.0278 on day 60 for 25 mice, and P = 0.0469 on day 75 for 15 mice). b Haematoxylin and Eosin histology of the paws of tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice 30 and 75 days after treatment with Diphtheria toxin and 1 µg of Poly(C) or Diphtheria toxin and 1 µg of VEGFC mRNA-LNPs intradermally. Poly(C) or VEGFC mRNA-LNPs were injected 8 days after Diphtheria toxin treatment. Representative images are shown of 5 mouse paws in each group (bars, 200 µm). c Paw fibroadipose area of tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice 30 and 75 days after treatment with Diphtheria toxin and 1 µg of Poly(C) or Diphtheria toxin and 1 µg of VEGFC mRNA-LNPs. Poly(C) or VEGFC mRNA-LNPs were injected 8 days after Diphtheria toxin treatment. Quantitative data are represented as mean and SEM in 8–9 mouse paws in each group stained with Haematoxylin and Eosin (two-tailed, paired T-test, P = 0.0001 on day 30 for 9 mice and P = 0.0047 on day 75 for 8 mice). d Anti-LYVE1, anti-Podoplanin immunofluorescent histology of the paws of tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice 30 and 75 days after treatment with Diphtheria toxin and 1 µg of Poly(C) or Diphtheria toxin and 1 µg of VEGFC mRNA-LNPs. Poly(C) or VEGFC mRNA-LNPs were injected 8 days after Diphtheria toxin treatment. Representative images are shown of 5 mouse paws in each group (bars, 50 µm). Arrows indicate LYVE1 and Podoplanin double positive lymphatic vessels. e Number of lymphatic vessels of tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice 30 and 75 days after the paw treatment with Diphtheria toxin and 1 µg of Poly(C) or Diphtheria toxin and 1 µg of VEGFC mRNA-LNPs. Poly(C) or VEGFC mRNA-LNPs were injected 8 days after Diphtheria toxin treatment. Quantitative data are represented as mean and SEM in 7–11 mouse paws in each group (two-tailed, paired T-test, P = 0.0024 after 30 days for 11 mice and P = 0.0008 after 75 days for 7 mice). f Anti-Collagen I immunofluorescent histology of the paws of tamoxifen-treated Flt4-CreERT2; iDTRfl/fl mice 30 and 75 days after treatment with Diphtheria toxin and 1 µg of Poly(C) or Diphtheria toxin and 1 µg of VEGFC mRNA-LNPs. Poly(C) or VEGFC mRNA-LNPs were injected 8 days after Diphtheria toxin treatment. Representative images are shown of 5 mouse paws in each group (bars, 50 µm). Asterisks indicate P < 0.05 compared with control. All cell nuclei are labeled with DAPI (blue) in paraffin-embedded tissues.