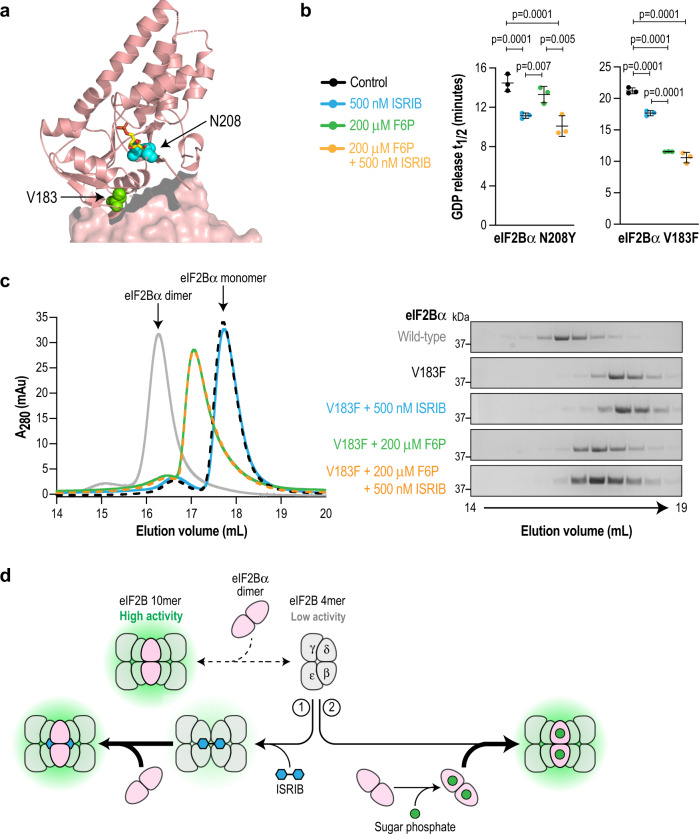
Fig. 5. F6P enhances decamer formation and activity of the VWM mutant eIF2BαV183F but not eIF2BαN208Y.
a Close-up view of a single eIF2Bα monomer showing the positions of residues V183 (green) and N208 (cyan). F6P is shown in stick representation. N208 is within the binding pocket and V183 is positioned at the interface with another eIF2Bα subunit. b GDP release t1/2 in a GEF assay using recombinant eIF2B reconstituted with either eIF2BαN208Y or eIF2BαV183F. N208Y activity is stimulated by ISRIB (blue) but not F6P (green), whereas the reverse is true for V183F. Both mutants are stimulated by the combination of ISRIB and F6P (orange). Bars are mean ± standard deviation of n = 3 independent experiments of 3 technical replicates each. Statistical significance was tested by one-way ANOVA with Tukey’s multiple testing correction. c Size-exclusion chromatography of purified recombinant wild-type or V183F eIF2Bα in the presence of ISRIB and/or F6P. UV absorbance chromatograms as well as Coomassie-stained fractions are shown. Data shown are representative of 3 independent replicates. Wild-type eIF2Bα is a dimer whereas eIF2BαV183F is a monomer, but is shifted towards a dimeric form by F6P. d Model depicting two distinct pathways to achieve eIF2B decamerization and activation. Arrow thickness indicates the rate of a reaction occurring. The synthetic activator ISRIB bridges the eIF2Bβ/δ interface to form an octamer, which then interacts with eIF2Bα2. Sugar phosphates bind to eIF2Bα2 and promote its interaction with eIF2B(βδγε) to form the holoenzyme.