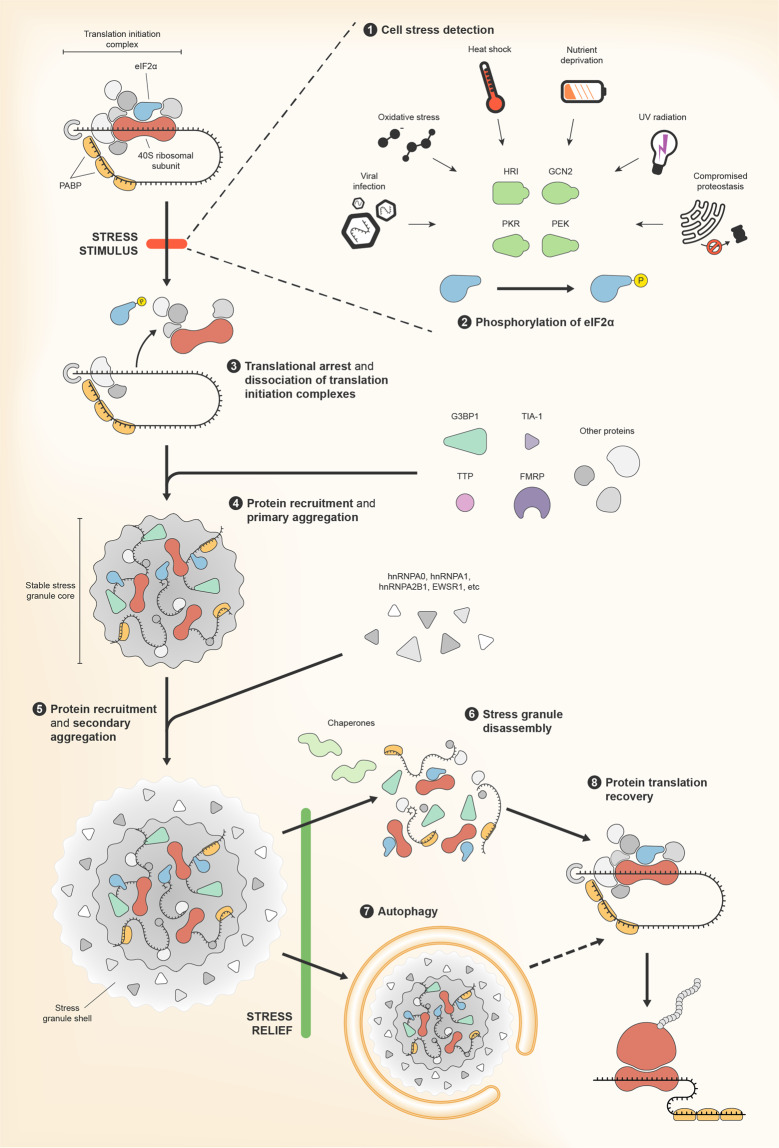
Fig. 2. The canonical stress granule assembly pathway.
(1) Formation of stress granules (SGs) can be triggered by diverse cell damaging conditions, including viral infection, oxidative stress, heat shock, nutrient deprivation, ultraviolet radiation or proteotoxic stress. Particular stress conditions are detected by specific kinases—protein kinase R (PKR), haem-regulated inhibitor (HRI), general control non-derepressible-2 (GCN2) and pancreatic eIF2α kinase (PEF)—that then become activated and (2) phosphorylate eukaryotic translation initiation factor 2 subunit alpha (eIF2α). (3) eIF2α is involved in the formation of translation initiation complexes and, when phosphorylated, leads to dissociation of these complexes and to translational arrest. (4) mRNAs, 40S ribosomal subunits, and proteins involved in translation start to accumulate and to assemble together, along with other proteins that are recruited to the forming SGs. This primary aggregation process produces a stable SG core. RNA-binding proteins (RBPs) that constitute SG cores include ras GTPase-activating protein-binding protein 1 (G3BP1), T-cell intracellular antigen-1 (TIA-1), tristetraprolin (TTP) and fragile X mental retardation protein (FMRP). (5) A secondary aggregation step resulting from additional, albeit weaker, intermolecular interactions originate the shell of the SGs. RBPs recruited in this step include heterogenous nuclear ribonucleoprotein A0 (hnRNPA0), hnRNPA1, hnRNPA2B1 and RNA-binding protein EWS (EWSR1). (6) When stress conditions abate, SGs are either disassembled by molecular chaperons or (7) are cleared by autophagy. (8) Disassembly allows for a rapid recovery of protein synthesis.