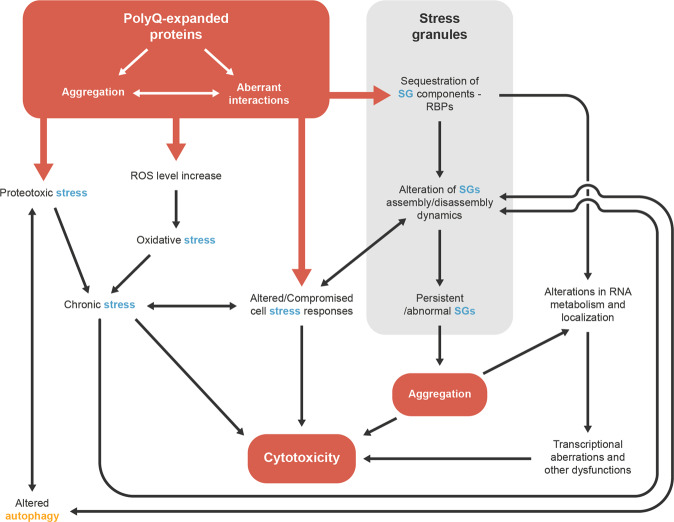
Fig. 4. The putative involvement of stress granules in the molecular pathophysiology of polyglutamine diseases.
Proteins bearing an expanded polyglutamine (PolyQ) tract display a tendency to aggregate and to engage in aberrant intermolecular interactions. Association of PolyQ-expanded proteins with stress granules (SGs) components, in particular RNA-binding proteins (RBPs) that are often prone to aggregate, may alter the dynamics of SGs assembly and disassembly. This can compromise SGs functionality, contributing to the globally deficient cell stress response that has been described to be a component of the molecular pathophysiology of PolyQ diseases. Oxidative stress (associated with an increase in reactive oxidative species - ROS - levels) and proteotoxic stress, which are known to result from expanded PolyQ protein expression, may culminate in a state of chronic cell stress, which may add to the abnormal SG assembly/disassembly dynamics and lead to the persistence of SGs. SGs formed under chronic stress are known to acquire abnormal properties and to seed toxic aggregation. Defects in autophagy caused by PolyQ protein expression may also contribute to the persistence of SGs and to their altered dynamics. Additionally, both the sequestration of RBPs to PolyQ aggregates and the toxic aggregation triggered by SGs may alter RNA metabolism and its subcellular localization, which in turn may lead to transcriptional aberrations. Chronic stress, a reduced ability to cope with cell stress, transcriptional alterations and a pernicious cascade of protein aggregation involving both the PolyQ-expanded proteins and the SGs may combine to produce the cytotoxic profile with is at the basis of cell dysfunction and loss, in PolyQ diseases.