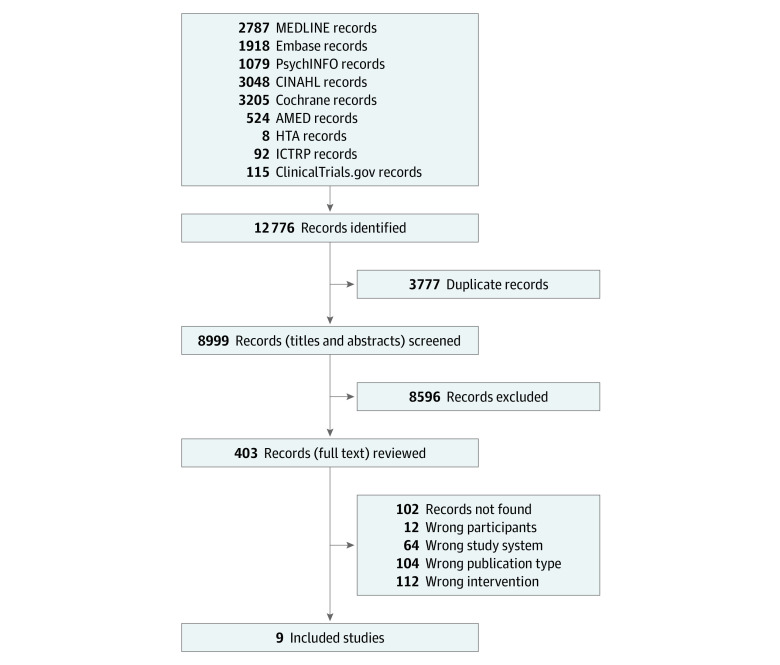
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart .
Search was conducted from the earliest record to March 4, 2019. AMED indicates Allied and Complementary Medicine Database; HTA, Health Technology Assessment; ICTRP, International Clinical Trials Registry Platform.