Abstract
OBJECTIVE:
While most cases of endometrial cancer can readily be classified as pure endometrioid, pure serous, or another type, others show an apparent mixture of serous and endometrioid components, or indeterminate serous versus endometrioid features. Since serous histology carries a worse prognosis than endometrioid, Gynecologic Oncology Group protocol GOG-8032 was established to examine whether the presence of a non-serous component is a favorable feature in an otherwise serous cancer.
METHODS:
934 women with serous cancer were prospectively identified among a larger group enrolled in GOG-0210. Six expert gynecologic pathologists classified each case as pure serous (SER, n=663), mixed serous and endometrioid (SER-EM-M, n=138), or indeterminate serous v. endometrioid (SER-EM-I, n=133) by H&E morphology. Follow-up data from GOG-0210 were analyzed.
RESULTS:
The subgroups did not differ on BMI, race, ethnicity, lymphovascular invasion, cervical invasion, ovary involvement, peritoneal involvement, omental involvement, FIGO stage, or planned adjuvant treatment. SER-EM-M patients were younger (p=0.0001) and less likely to have nodal involvement (p=0.0287). SER patients were less likely to have myoinvasion (p=0.0002), and more likely to have adnexal involvement (p=0.0108). On univariate analysis, age, serous subtype, race, and components of FIGO staging predicted both progression-free and overall survival. On multiple regression, however, serous subtype (SER, SER-EM-M, or SER-EM-I) did not significantly predict survival.
CONCLUSIONS:
There were few clinicopathologic differences between cases classified as SER, SER-EM-M, and SER-EM-I. Cases with a mixture of serous and endometrioid morphology, as well as cases with morphology indeterminate for serous v. endometrioid type, had the same survival as pure serous cases.
Keywords: Endometrial carcinoma, endometrioid carcinoma, serous carcinoma, malignant mixed tumors, pathology, survival analysis
INTRODUCTION
Endometrial carcinoma is the most common malignant neoplasm of the female genital tract and is increasing in incidence, with an estimated 61,880 cases diagnosed in 2019, causing 12,160 deaths [1]. There has been a dramatic increase in our understanding of uterine cancer in the past 40 years, but the overall survival (OS) rates have not improved appreciably. While only three types of endometrial carcinoma were recognized in the 1970s (adenocarcinoma not otherwise specified, adenoacanthoma, and mesonephric carcinoma), more than a dozen types and subtypes are currently recognized, several of which have markedly different behavior. The histologic type or cell type of an endometrial tumor can, along with other prognostic factors, be relevant in determining the need for staging procedures and the likely benefit of adjuvant treatment.
Serous carcinoma is one of those types of endometrial carcinoma that was not recognized or appropriately staged in the past. Serous carcinoma or papillary carcinoma with psammoma bodies was rarely described as isolated case reports in pathology publications in the earlier 20th century, but it was not until 1983 that Eifel et al. alerted the medical community to the aggressive behavior of a relatively unusual tumor that they referred to as papillary serous carcinoma [2], with OS as low as 60%. Numerous other investigators subsequently expanded these observations [3–9] and confirmed that serous carcinoma has more aggressive behavior than stage-matched high-grade endometrioid carcinoma [10]. Serous carcinoma of the endometrium is composed of cells bearing a close resemblance to high-grade serous carcinoma of the ovary, which is the most common subtype of epithelial ovarian cancer and of ovarian cancer overall. While many serous uterine carcinomas are composed entirely of this one cell type, some serous carcinomas are admixed with endometrioid [11] or clear cell carcinoma, and others have a histologic appearance with some features of serous carcinoma and others of endometrioid or clear cell morphology. The biology of these mixed or indeterminate tumors (also described as “morphologically ambiguous” [12]) has not been well characterized, and it has not been clear which subtype drives the behavior in such cases. While the World Health Organization classification of tumors includes a mixed category, a 10% admixture of each component is required to meet the definition, meaning that tumors with a lesser admixture are excluded.
The Cancer Genome Atlas analysis of endometrial cancer revealed the existence of four molecular subtypes of endometrial cancer, among a cohort of cases histologically classified as endometrioid or serous. These subtypes included POLE mutant (ultramutated), microsatellite unstable (MSI, hypermutated), copy number-low (CN-low, endometrioid-like) and copy number-high (CN-high, serous-like) tumors [13]. Tumors with serous histology fall almost exclusively into the CN-high group [13]. An algorithm based on POLE sequencing, mismatch repair testing, and p53 immunohistochemistry (ProMisE [14]) has been shown to serve as a surrogate method for classifying endometrial cancer into these four types. Endometrial serous cancers consistently have aberrant p53 expression by immunohistochemistry [15], thereby falling into a “p53 abnormal” category that corresponds to the CN-high TCGA group. While the ProMisE classifier may become an important aspect of endometrial cancer care [16,17], it does not specifically address the handling of cases with mixed histology.
The present study is intended to provide information about the behavior of tumors with a serous component admixed with endometrioid components. In order to better understand the differences in epidemiology, genetic alterations, biologic behavior, patterns of spread, stage at diagnosis, frequency of recurrence, and disease-specific survival among different types of endometrial carcinoma, a subcommittee of the Gynecologic Oncology Group (GOG) created a multi-disciplinary protocol in 2001. In this study (GOG-210), a very large cohort of women with various types of endometrial carcinoma completed a thorough epidemiologic evaluation, and then were initially treated in identical fashion including hysterectomy, salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy. A number of these women had either pure serous carcinoma (SER), serous carcinoma mixed with a definite endometrioid component (SER-EM-M), or carcinoma with features that were ambiguous between serous and endometrioid (“indeterminate” cell type, SER-EM-I), based on histologic classification. Sub-protocol GOG-8032 was established to report on the clinical and pathologic characteristics of these women, with the aim of determining whether these typical and variant subtypes of serous carcinoma present at significantly different stages or have different recurrence or survival rates.
Materials and Methods
Inclusion and Exclusion Criteria and Treatment:
Patients were enrolled in GOG-210, “A Molecular Staging Study of Endometrial Carcinoma,” between September 22, 2003 and December 1, 2011. The study was approved by the Institutional Review Board of the participating institutions. The protocol was amended on September 24, 2007 to enhance accrual among selected sub-populations by restricting eligibility to high-risk cell types and underrepresented minorities. All women with a biopsy or curettage diagnosis of endometrial carcinoma were asked to complete an epidemiologic questionnaire, and were initially treated by total hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy, with collection of serum and urine, as well as fresh-frozen and formalin-fixed neoplastic and non-neoplastic tissues for other investigations. Adjuvant therapy and the type of treatment for metastatic or recurrent disease were left to the discretion of the treating physician and patient.
Extended Central Pathologic Review:
The pathologist at each institution was asked to review the material from the hysterectomy specimen and report, and select and provide recut slides for central review depicting pertinent pathologic characteristics including histologic type, cervical involvement and any sites of metastasis. Lymphovascular space invasion, depth of invasion, and cytologic material were not reviewed. Cases were initially reviewed by rotating groups of pathologists attending semiannual GOG meetings. After comparing the central review data on these cases with that from the submitting institution, it was determined that the reproducibility in assessment was sufficiently great that this review was not necessary for endometrioid, adenosquamous and mucinous carcinomas, grades 1 and 2, stages IA-IC (FIGO 1988). Under GOG-8032, all cases with other histologies were submitted for an extended central pathologic review that was carried out at double-headed microscopes by rotating pairs of a group of 6 pathologists (“G6”: OI, KP, GR, MS, RS, RZ), following an extended discussion and recording of mutually agreed-upon criteria for diagnoses and interpretations of findings. All G6 reviews were documented on a standardized review form and reflected the consensus opinion of two G6 members whose initials were recorded on the form. This methodology was designed to maximize standardization of criteria across the G6 pathologists, but did not provide any practical way to measure interobserver variability, since there were no individual observations by any single pathologist. When extended central pathologic (G6) review was not available, the results of central pathology (GOG) review or local institutional pathology review were substituted, in order to minimize missing values.
Cell Type Definitions:
Serous carcinoma was defined per Crum and Lee’s Diagnostic Gynecologic and Obstetric Pathology [18]. Based on published literature and prior experience, the G6 determined that while some cases of serous carcinoma were composed purely of the individual cell type, others fell into a mixed or indeterminate group. Therefore, categories of pure serous carcinoma (SER), mixed serous and endometrioid carcinoma (SER-EM-M), and indeterminate serous v. endometrioid carcinoma (SER-EM-I) were used (Figure 1). The “mixed” category was used for tumors that displayed two or more well-defined patterns of neoplasm. The “indeterminate” category was used for tumors that displayed a single pattern that had a mixture of features that did not fit neatly into any of the cell types. Tumor classification was performed primarily on histologic grounds, supported by any immunohistochemical data provided to the panel in the original pathology report. Immunostained slides were not generally available.
Figure 1.
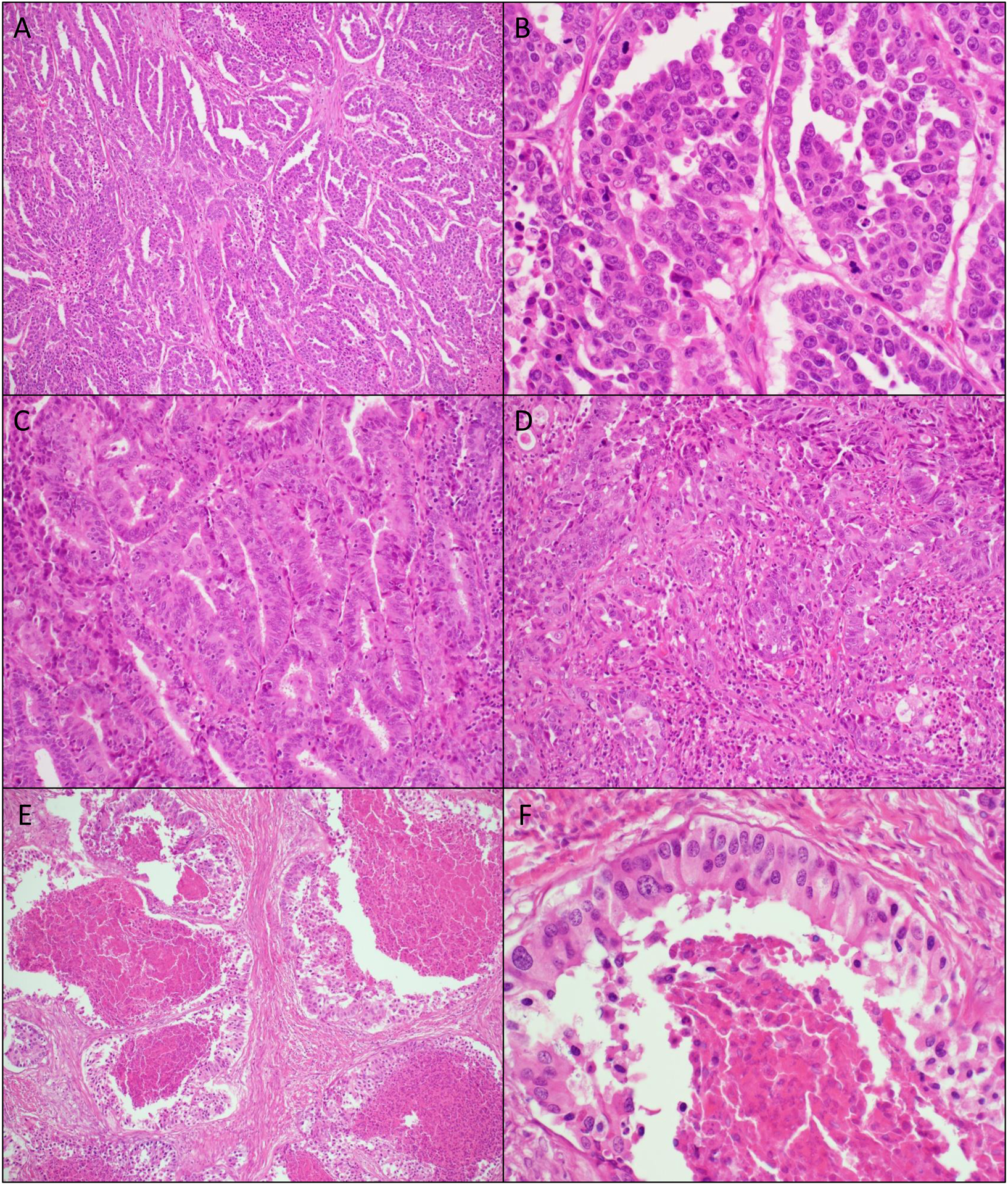
Pure serous endometrial cancer (SER), (A) 100x and (B) 400x original magnification.
Mixed serous and endometrioid endometrial cancer (SER-EM-M), as determined by histologic review by the G6 pathologists. A single case shows both (C) endometrioid-like areas and (D) serous-like areas, 200x original magnification. The term “mixed” was applied when disparate cell types could be identified.
Endometrial cancer with features intermediate between endometrioid and serous, so-called indeterminate cancer (SER-EM-I) as determined by the G6 pathologists. H&E examination at (E) 100x and (F) 400x shows cancer with a single morphology, with cells sharing some features of serous carcinoma (high-grade nuclei, high mitotic rate) and endometrioid carcinoma (tall columnar cells).
Statistical Considerations:
SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses. All statistical tests were two-sided, and 0.05 was used as a significance level to classify individual statistical test results as significant. No adjustment was made for multiple tests. All observations with missing values (including not reported/not assessed) were excluded in statistical analyses.
The relationship between the three serous histology subtypes (i.e., SER, SER-EM-M, and SER-EM-I) and each of the candidate baseline patient or clinicopathologic characteristics was evaluated by either Monte-Carlo permutation-based exact chi-square tests for discrete-type characteristics, or Monte-Carlo permutation-based exact Kruskal-Wallis tests for interval-type characteristics.
Progression-free survival (PFS) and OS were compared among the three serous subtypes by log-rank tests. PFS was defined as the duration of time from study entry to date of disease recurrence or progression, death, or the date of last contact, whichever occurred first. PFS was censored in patients who were alive and had not experienced disease progression or recurrence at last contact. OS was defined as the duration of time from study entry to the time of death due to any cause or the date of last contact. In addition, PFS and OS were examined for the selected baseline characteristics (whenever feasible) by log-rank tests. A Cox proportional hazards model was used to estimate the corresponding hazard ratios. The associations between PFS/OS and serous subtype were further assessed by Cox proportional hazards multiple regression with adjustment for age, race, myometrial invasion, and FIGO stage. Due to small numbers, patients with race other than black or white were not included in the survival analysis.
RESULTS
A total of 6,124 patients were enrolled in GOG-0210. After eliminating those with ineligible cell types, primary sites, pretreatment or other factors, 5,866 were eligible for further analysis (Supplemental Table 1).
Among the 5,866 eligible patients, 3,715 (63.3%) were enrolled during the initial, unrestricted period of enrollment. In the general population of women with endometrial carcinoma enrolled in this study, approximately 11% had a pure, mixed or indeterminate serous carcinoma. During the unrestricted period, there were 273 women with SER (SER; 7.3% ), 91 mixed serous with endometrioid carcinoma (SER-EM-M; 2.4%), 17 mixed serous with clear cell carcinoma (SER-CC-M; 0.5%), 59 indeterminate serous v. endometrioid carcinoma (SER-EM-I; 1.6%), and 3 indeterminate serous v. clear cell carcinoma (SER-CC-I; 0.1%) enrolled on this protocol (Table 1). Analysis of the tumors with clear cell morphology will be reported separately.
Table 1.
Distribution of serous tumor histology by enrollment period and extended central pathology (G6) review status
| Enrollment period | G6 review status | ||||||
|---|---|---|---|---|---|---|---|
| Tumor histology | Unrestricted | Restricted | Yes | No | Total | ||
| Serous, pure (SER) | 273 | 390 | 644 | 19 | 663 | ||
| Mixed serous and endometrioid (SER-EM-M) | 91 | 47 | 138 | 0 | 138 | ||
| Indeterminate serous v. endometrioid (SER-EM-I) | 59 | 74 | 132 | 1 | 133 | ||
| Mixed serous and clear cell (SER-CC-M) | 17 | 35 | 52 | 0 | 52 | ||
| Indeterminate serous and clear cell (SER-CC-I) | 3 | 18 | 21 | 0 | 21 | ||
| Total | 443 | 564 | 987 | 20 | 1,007 | ||
Among other relatively uncommon characteristics, there was a desire to enrich the study population for high-risk histologies, including serous carcinoma, leading to the decision to carry out a restricted phase of the study during which the protocol remained open to any woman with serous carcinoma. This phase resulted in enrollment of 390 additional pure serous cases, 47 SER-EM-M, and 74 SER-EM-I. Ultimately, 3,566 cases out of the 5,866, including nearly all cases submitted as serous carcinoma, were reviewed by the G6. The final accrual included 663 pure serous carcinomas, 138 mixed serous and endometrioid carcinomas, 133 indeterminate serous or endometrioid carcinomas, 52 mixed serous and clear cell carcinomas, and 21 indeterminate serous or clear cell carcinomas, for a total of 1,007 cases (Table 1).
A series of comparisons were undertaken among SER, SER-EM-M, and SER-EM-I, in order to determine whether the presence of endometrioid components or features correlated with other clinicopathologic characteristics (Table 2). Generally, the three groups SER, SER-EM-M, and SER-EM-I had similar clinicopathologic features, and did not differ significantly by FIGO stage (FIGO 1988) at 0.05 significance level. However, patients with SER-EM-M were slightly younger (p=0.0001) than the other two groups, and less likely to show any nodal involvement (p=0.0287). Patients with SER were less likely to have myometrial invasion (p=0.0002) than either SER-EM-M or SER-EM-I, and more likely to have involvement of any adnexal site (p=0.0108). Planned adjuvant therapy was dichotomized (chemotherapy and/or radiotherapy versus none) and was not different between groups (p-0.1206). Log-linear models with adjustment of the correlation between serous subtype and enrollment period were utilized to examine these relationships, and the results (not shown) were consistent with these findings at the 0.05 significance level.
Table 2.
Distribution of baseline characteristics by serous histology subtype for all eligible patients regardless of enrollment period.
| SER | SER-EM-M | SER-EM-I | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||||
| Age (years) | 0.0001 | |||||||||||
| BMI (kg/m2) | 0.0574 | |||||||||||
| Race | 0.0771 | |||||||||||
| Ethnicity | 0.5266 | |||||||||||
| Myometrial invasion | 0.0002 | |||||||||||
| Lymphovascular invasion | 0.3264 | |||||||||||
| Cervical invasion | 0.2982 | |||||||||||
| Adnexal involvement | 0.0108 | |||||||||||
| Nodal involvement | 0.0287 | |||||||||||
| Peritoneal involvement | 0.1923 | |||||||||||
| Omental involvement | 0.3247 | |||||||||||
| FIGO stage | 0.1741 | |||||||||||
| Planned adjuvant therapy | 0.1206 | |||||||||||
| Total | 663 | 71.0% | 138 | 14.8% | 133 | 14.2% | 934 | 100.0% | ||||
NA/NR, not available/not reported;
CT, chemotherapy; RT, radiotherapy
We then considered the possibility that differences in biologic behavior (e.g., tumor aggressiveness) might result in a difference in outcomes for the three histologic subtypes. As of January 15, 2019, the overall median follow-up time for vital status was 106.9 months (122.5 months for unrestricted period and 91.5 months for restricted period, respectively) in patients with histology of SER, SER-EM-M or SER-EM-I. Survival analyses including PFS and OS between the three serous subtypes were performed, initially by univariate analysis. At the 0.05 significance level, PFS was significantly different among the three serous subtypes: SER-EM-M had better PFS compared to either SER (hazard ratio [HR]: 0.68, 95% confidence interval [CI]: 0.51 – 0.88) or SER-EM-I (HR: 0.65, 95% CI: 0.46 – 0.91) (p=0.0131 by log-rank test) (Table 3). Results from additional log-rank tests indicated that progression-free survival was significantly worse with higher age dichotomized by median, race of African-American vs. White, incrementally deeper myoinvasion, presence of LVSI, presence of cervical stromal invasion, adnexal involvement, nodal involvement, or incremental FIGO stage, separately. Similar results were found for OS (Table 3). In particular, univariate analysis showed that SER-EM-M had better OS compared to either SER (HR: 0.72, 95% CI: 0.54 – 0.95) or SER-EM-I (HR: 0.66, 95% CI: 0.47 – 0.93) (p=0.0408 by log-rank test).
Table 3.
Univariate analysis of progression-free and overall survival in all eligible serous patients
| Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| P-value1 | Hazard Ratio2 | 95% Confidence Interval | P-value1 | Hazard Ratio2 | 95% Confidence Interval | ||
| Enrollment period | Restricted vs Unrestricted | 0.4639 | 0.937 | 0.7865 to 1.1162 | 0.9413 | 1.007 | 0.8386 to 1.2100 |
| Serous subtype | SER-EM-I vs SER | 0.0131 | 1.041 | 0.8095 to 1.3200 | 0.0408 | 1.092 | 0.8417 to 1.3968 |
| SER-EM-M vs SER | 0.677 | 0.5112 to 0.8802 | 0.723 | 0.5414 to 0.9470 |
|||
| SER-EM-M vs SER-EM-I | 0.650 | 0.4636 to 0.9081 | 0.662 | 0.4673 to 0.9333 | |||
| Age (years) | High (> median) vs low (≤ median) | <0.0001 | 1.481 | 1.2447 to 1.7670 | <.0001 | 1.571 | 1.3096 to 1.8880 |
| BMI (kg/m2) excluding < 18.5 | 18.5 ≤ BMI < 25 vs 25 ≤ BMI < 30 | 0.8101 | 1.085 | 0.8287 to 1.4188 | 0.3525 | 1.150 | 0.8666 to 1.5255 |
| 18.5 ≤ BMI < 25 vs 30 ≤ BMI < 35 | 0.981 | 0.7529 to 1.2744 | 0.920 | 0.6998 to 1.2069 | |||
| 25 ≤ BMI < 30 vs 30 ≤ BMI < 35 | 0.904 | 0.6998 to 1.1661 | 0.800 | 0.6109 to 1.0457 | |||
| BMI ≥ 35 vs 18.5 ≤ BMI < 25 | 1.032 | 0.8081 to 1.3248 | 1.063 | 0.8247 to 1.3764 | |||
| BMI ≥ 35 vs 25 ≤ BMI < 30 | 1.120 | 0.8836 to 1.4246 | 1.222 | 0.9522 to 1.5760 | |||
| BMI ≥ 35 vs 30 ≤ BMI < 35 | 1.012 | 0.8033 to 1.2787 | 0.978 | 0.7699 to 1.2452 | |||
| Race | Black/African American vs White | 0.0149 | 1.289 | 1.0463 to 1.5767 | 0.0106 | 1.318 | 1.0616 to 1.6240 |
| Ethnicity | Hispanic vs Non-Hispanic | 0.5798 | 1.132 | 0.7075 to 1.7078 | 0.7118 | 1.093 | 0.6568 to 1.7001 |
| Prior cancer | No vs Yes | 0.9162 | 1.013 | 0.8019 to 1.2966 | 0.7725 | 0.964 | 0.7582 to 1.2433 |
| Myoinvasion | Inner Half vs None | <.0001 | 1.567 | 1.2185 to 2.0304 | <.0001 | 1.569 | 1.2028 to 2.0652 |
| Inner Half vs Outer Half/Serosa | 0.489 | 0.4011 to 0.5958 | 0.465 | 0.3781 to 0.5699 | |||
| None vs Outer Half/Serosa | 0.312 | 0.2419 to 0.3994 | 0.296 | 0.2263 to 0.3834 | |||
| Lymphovascular space invasion | No vs Yes | <.0001 | 0.429 | 0.3586 to 0.5127 | <.0001 | 0.429 | 0.3561 to 0.5164 |
| Cervix invasion | No/Glandular vs Stromal (S)/Both | <.0001 | 0.461 | 0.3805 to 0.5612 | <.0001 | 0.470 | 0.3853 to 0.5763 |
| Adnexal involvement | No vs Yes | <.0001 | 0.374 | 0.3080 to 0.4570 | <.0001 | 0.394 | 0.3223 to 0.4847 |
| Nodal involvement | No vs Yes | <.0001 | 0.370 | 0.3073 to 0.4454 | <.0001 | 0.387 | 0.3198 to 0.4700 |
| Peritoneal involvement | No vs Yes | <.0001 | 0.332 | 0.2595 to 0.4284 | <.0001 | 0.345 | 0.2681 to 0.4490 |
| Omental involvement | No vs Yes | <.0001 | 0.339 | 0.2716 to 0.4278 | <.0001 | 0.367 | 0.2919 to 0.4656 |
| Other pelvic involvement | No vs Yes | <.0001 | 0.410 | 0.3221 to 0.5270 | <.0001 | 0.451 | 0.3509 to 0.5857 |
| Other abdominal involvement | No vs Yes | <.0001 | 0.374 | 0.2784 to 0.5131 | <.0001 | 0.415 | 0.3053 to 0.5769 |
| FIGO stage | I vs II | <.0001 | 0.599 | 0.4362 to 0.8396 | <.0001 | 0.592 | 0.4255 to 0.8391 |
| I vs III | 0.345 | 0.2792 to 0.4246 | 0.358 | 0.2870 to 0.4450 | |||
| I vs IV | 0.175 | 0.1362 to 0.2271 | 0.185 | 0.1424 to 0.2413 | |||
| II vs III | 0.575 | 0.4140 to 0.7819 | 0.605 | 0.4303 to 0.8313 | |||
| II vs IV | 0.293 | 0.2050 to 0.4121 | 0.313 | 0.2165 to 0.4447 | |||
| III vs IV | 0.509 | 0.4019 to 0.6491 | 0.517 | 0.4051 to 0.6643 | |||
Log-rank test,
Estimated by Cox proportional hazards model
Based upon the results of univariate analysis, Cox proportional hazards multiple regression methods were used to further evaluate the association of the three serous subtypes with PFS or OS after adjustment for age, race, myoinvasion and FIGO stage. With these adjustments, there was no significant difference between the 3 serous subtypes for PFS and OS, as the hazard ratios were not significantly different from 1 (Table 4). The other covariates in the model did show significant and independent associations with survival. Specifically, after adjustment for the other variables, lower FIGO stage was associated with a hazard ratio <1 compared to patients with a higher stage, indicating better PFS and OS. Similarly, having less myometrial invasion was associated with better PFS and OS compared to a patient with more myometrial invasion. African-American patients had worse PFS and OS compared to white patients. Older patients had worse PFS and OS compared to younger patients.
Table 4.
Cox proportional hazards multiple regression analysis of progression-free and overall survival in all eligible serous carcinoma patients with white or black race.
| Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Comparison | P-value1 | Hazard Ratio | 95% Confidence Interval | P-value1 | Hazard Ratio | 95% Confidence Interval |
| Serous subtype | SER vs SER-EM-I | 0.1304 | 1.074 | 0.8372 to 1.3963 | 0.2859 | 1.061 | 0.8198 to 1.3942 |
| SER vs SER-EM-M | 1.333 | 1.0146 to 1.7819 | 1.259 | 0.9525 to 1.6951 | |||
| SER-EM-I vs SER-EM-M | 1.242 | 0.8781 to 1.7619 | 1.187 | 0.8307 to 1.7010 | |||
| Age (years) | Unit=10 | <0.0001 | 1.340 | 1.2080 to 1.4873 | <0.0001 | 1.458 | 1.3064 to 1.6269 |
| Race group | Black/African American vs White | 0.0011 | 1.425 | 1.1488 to 1.7553 | 0.0013 | 1.433 | 1.1462 to 1.7780 |
| Myoinvasion | None vs Inner Half | <0.0001 | 0.720 | 0.5499 to 0.9349 | <0.0001 | 0.715 | 0.5375 to 0.9440 |
| Inner Half vs Outer Half/Serosa | 0.623 | 0.5044 to 0.7675 | 0.607 | 0.4874 to 0.7538 | |||
| None vs Outer Half/Serosa | 0.448 | 0.3394 to 0.5868 | 0.434 | 0.3236 to 0.5766 | |||
| FIGO stage | I vs II | <0.0001 | 0.703 | 0.5072 to 0.9936 | <0.0001 | 0.703 | 0.5004 to 1.0073 |
| I vs III | 0.464 | 0.3689 to 0.5835 | 0.462 | 0.3634 to 0.5877 | |||
| I vs IV | 0.245 | 0.1853 to 0.3248 | 0.253 | 0.1895 to 0.3391 | |||
| II vs III | 0.660 | 0.4705 to 0.9077 | 0.658 | 0.4626 to 0.9154 | |||
| II vs IV | 0.348 | 0.2396 to 0.4987 | 0.360 | 0.2446 to 0.5214 | |||
| III vs IV | 0.527 | 0.4090 to 0.6841 | 0.547 | 0.4217 to 0.7142 | |||
Based on a type 3 test for a Cox proportional hazards model with the covariates of serous subtype, age, race, myoinvasion and FIGO stage
To illustrate the lack of significant association between serous subtype and survival, predicted survival curves were constructed based on the Cox proportional hazards model, using adjustments for age (67 years), race (white) and myometrial invasion (inner half). The results show that a patient with a mixed or indeterminate serous tumor (SER-EM-M or SER-EM-I) had an expected PFS (Figure 2A) and OS (Figure 2B) that was not different from a patient with a pure serous tumor (SER).
Figure 2.
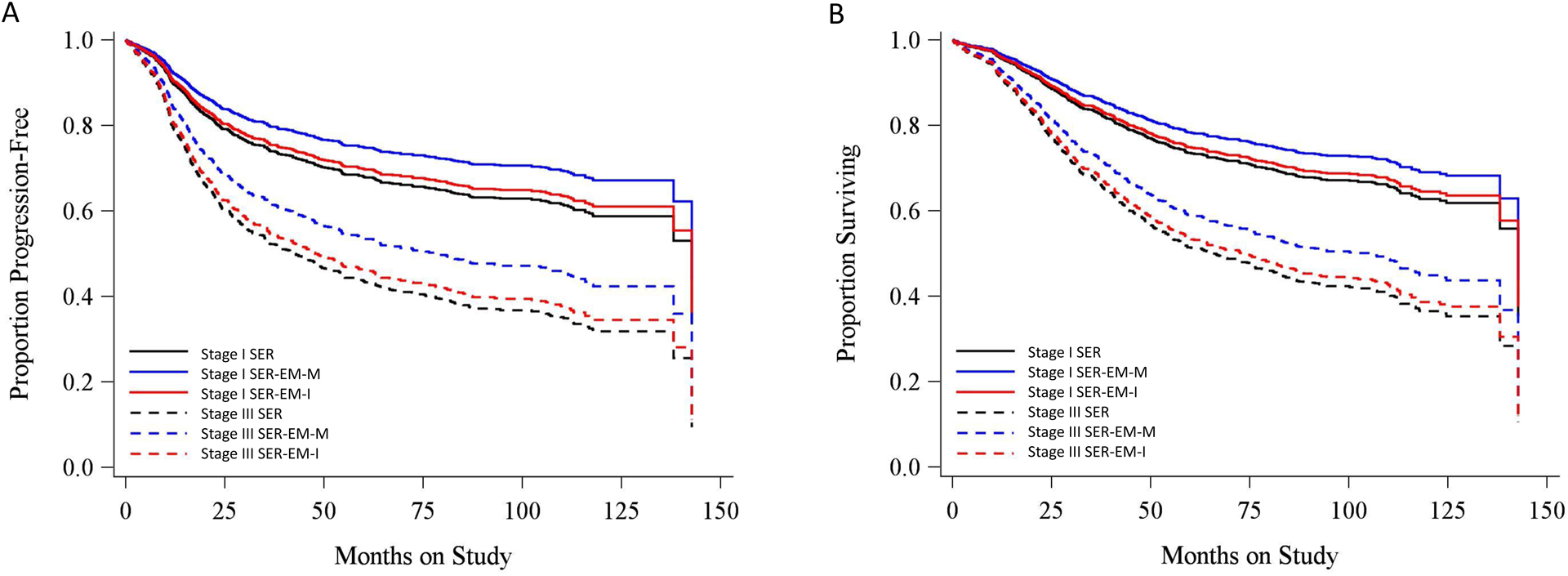
Predicted (A) progression-free and (B) overall survival of patients with three subtypes of serous cancer for stage I vs stage III based on a Cox PH model adjusted for age (67 years), race (white), and myometrial invasion (inner half).
DISCUSSION
This study takes advantage of data from GOG-210, a large series of well-characterized endometrial cancer cases with homogeneous initial surgical treatment, to investigate the significance of subtypes of serous carcinoma. Specifically, pathologists have observed that some serous carcinomas appear histologically “pure” (SER), some are admixed with endometrioid elements (SER-EM-M), whereas others have features that appear indeterminate for serous versus endometrioid adenocarcinoma and cannot easily be assigned to one or the other category (SER-EM-I). The latter group is sometimes reported by pathologists using terms such as “endometrioid carcinoma with serous features”. Analysis of baseline characteristics showed that the three groups were similar, differing only in age, myometrial invasion, adnexal involvement, or nodal involvement. Although marginally significant differences in PFS or OS were observed by log-rank tests among these 3 serous subtype patients, further Cox proportional hazards multiple regression showed no significant difference in PFS or OS for patients with these three histologic subtypes after adjustment for age, race, myoinvasion and FIGO stage.
The tumor types described here as SER-EM-I and SER-EM-M do not seem to be rare—in GOG 210, they accounted for 271 out of 974 carcinomas that had any serous component—yet they have been discussed only rarely in the literature. Boruta et al. examined the significance of a serous component in grade 3 endometrioid carcinomas, and reported that tumors with more than a 50% serous component had a significantly worse PFS and OS than pure endometrioid tumors. They did not find evidence of a direct relationship between serous percentage and survival of cases presenting at either early or late stage, suggesting that mixed serous and endometrioid cases have the same adverse prognosis as pure serous cases [11], analogous to our findings. Smaller studies have reached variable conclusions about the prognosis of tumors with mixed or indeterminate serous and endometrioid histology [4,19].
Strengths of the study included its large size, with more than 133 patients in each of the groups, making it likely that clinically significant differences between groups would be discovered, if present. The six pathologists responsible for case classification were internationally recognized expert gynecologic pathologists, practicing in different regions and countries, who undertook extensive discussion to define the categories for this study. The study also benefited from a lengthy follow-up interval.
This study does have several weaknesses. Cases were classified as SER, SER-EM-M, or SER-EM-I based on representative slides submitted by enrolling institutions. The central reviewers would not have been able to document any histologic components not present on these slides. This protocol—which was activated in 2003—used H&E-stained slides as the basis for classification. There was no protocolized use of immunostains, such as p53 or p16, that could support a diagnosis of serous versus endometrioid adenocarcinoma [7], although source institutions may have used such stains. In addition, the study did not use molecular correlates, such as TP53 mutation status, to support the classification.
Given the subjective nature of histologic diagnosis, there could be disagreement about the classification of any particular case. We believe the extended central pathologic review will have minimized any systematic error in the classification process. Nonetheless, a limitation is that there was no formal assessment of interobserver reproducibility. Studying the performance of the SER, SER-EM-M, and SER-EM-I categories outside the G6 group of pathologists was not in the scope of this protocol, but could be explored in the future.
Another limitation of the study is that the relative percentage of each component in the mixed serous and endometrioid cases was not recorded, as this is inherently impossible when only representative slides are examined. We therefore have no ability to determine whether there is a relation between percent serous carcinoma and clinicopathologic characteristics. In current clinical practice, cases with any admixture of serous carcinoma are often managed as serous. Supporting this approach, we found that cases classified as SER-EM-M had predicted survival that was not different from pure SER cases.
Treatment represents a potential confounding variable, if tumors of different serous subtypes received different adjuvant therapy. The protocol collected data on initial adjuvant plans, and there was no difference in intent to give adjuvant therapy (dichotomized as any vs. none) between serous subtypes. We do not know if there were differences in actual initial regimen, subsequent therapy or response. We also do not know the treating physician’s motivations in choosing adjuvant therapy or whether factors such as the percentage of the serous component were taken into account. Practice guidelines prevailing during the study period did not make different treatment recommendations for serous versus high-grade endometrioid cancer, nor for SER, SER-EM-M, and SER-EM-I subtypes.
By design, GOG-0210 included only patients with residual endometrial cancer at hysterectomy, whose resection material was available for central pathology review. The study is therefore biased towards patients with a larger burden of disease. Our data are not specifically informative as to patients with more limited disease, such that it is seen only on biopsy or curettage, with no residual cancer at hysterectomy. Such cases account for up to one-fifth of endometrial cancers overall [2,20], and have previously been reported to have a good prognosis, including in serous cases (although this subgroup does not seem to have been extensively analyzed).
As shown in Table 1, case review by the G6 pathologists resulted in a number of cases being classified as mixed serous and clear cell (SER-CC-M) or indeterminate serous and clear cell (SER-CC-I); there were also 136 cases classified as pure clear cell carcinoma. GOG-0210 also included 3,657 patients with pure endometrioid adenocarcinoma. For clarity, comparisons among these groups, and between these groups and the serous subtypes presented in the present article, will be reported in a separate contribution.
Our results generally show that SER, SER-EM-M and SER-EM-I do not differ for the outcomes we have measured, but still allow for the possibility that favorable-prognosis subgroups may be harbored within the category of serous tumors; indeed, this is likely, as has been shown for “serous” carcinomas in young patients, some of which belonged to the mismatch-repair deficient and POLE-mutated TCGA subtypes when further classified by ancillary methods [21]. Similarly, p53 immunostaining allows for clinically informative prognostication in morphologically ambiguous serous-like cancers [12]. Our data do indicate that endometrioid-like morphology or histologic components are not, in themselves, sufficient to identify such a subgroup.
In the years since this protocol was designed, progress in endometrial cancer research has suggested ways in which a non-serous tumor may mimic serous, mixed or indeterminate histology. POLE-mutated carcinomas carry a high burden of mutations and may show mixed or ambiguous serous features. MMR-deficient tumors may appear heterogeneous and may acquire p53 mutations within a subclone [22]. Tumors of fundamentally endometrioid nature may thus secondarily acquire a serous phenotype, which appears to be especially common in young patients (<60 years) with a diagnosis of serous carcinoma [21]. In this regard we note that SER-EM-M tumors presented at a younger age than the other groups in our study. There is also a subset of patients whose tumors harbor features of both serous and endometrioid adenocarcinoma despite lacking MMR deficiency or POLE mutation [21].
In conclusion, we report here a prospective clinicopathologic analysis of patients with pure serous carcinoma of the endometrium, as compared with patients with serous carcinoma having a mixed or indeterminate endometrioid component by H&E. Neither baseline characteristics nor survival were different, arguing that an apparent endometrioid component within a serous tumor, as identified by H&E examination, does not place the tumor in a clinically different subcategory. These data would support managing any tumor that appears morphologically to be a mixture of endometrioid and serous carcinoma in the same manner as a pure serous carcinoma, unless ancillary studies suggest a more prognostically favorable subclassification.
Supplementary Material
RESEARCH HIGHLIGHTS.
663 pure uterine serous cancers were compared with 138 mixed serous-endometrioid and 133 indeterminate cases
Pure, mixed and indeterminate serous carcinomas had similar clinicopathologic characteristics
On multiple regression, the presence of an endometrioid component did not independently predict survival
Acknowledgments
This study was supported by National Institute of Health grants to NRG Oncology (1 U10 CA180822) and NRG Operations (U10CA180868), NCORP (UG1CA189867), NRG Specimen Bank (U24CA196067), and was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
CONFLICTS OF INTEREST
Dr. Hagemann served as a consultant for Change Healthcare related to molecular pathology.
He also provided expert witness services to multiple clients regarding gynecologic and breast cases.
Dr. Powell reports personal fees from Tesaro, Merck, Roche/Genentech, Clovis Oncology, AstraZeneca, Johnson & Johnson, Eisai and Abbvie.
Dr. Gunderson’s institution received money for consultancy from Agenus, Cordgenics, Leap, Clovis, and GSK.
Dr. Mathews reports grants from Syros, Deciphera, AstraZeneca, Astellas Pharma, Tesaro/GSK, Seattle Genetics and Regeneron outside the submitted work.
Dr. Pearl received a grant from the Gynecologic Oncology Group. He also has grants/grants pending from GOG/NIH. He has received Royalties from Vivitek, Inc. via SBU Research Foundation.
Dr. Alvarez Secord reports grants from AbbVie, Amgen, AstraZeneca, Clovis, Astellas Pharma Inc., Boehringer Ingelheim, Briston Meyers Squibb, Eisai, Endocyte, Exelixis, Incyte, Merck, PharmaMar, Immutep Ltd., Roche/Genentech, Seattle Genetics, Inc., Tesaro/GSK, VBL Therapeutics, National Cancer Trial Network; honoraria from Aravive, AstraZeneca, Clovis, Cordgenics, Eisai, Janssen/Johnson & Johnson, Merck, Mersana, OncoQuest, Roche/Genentech, Tesaro/GSK Advisory Boards; participation on Clinical Trial Steering Committees (uncompensated) for Roche/Genentech and VBL Therapeutics; and member of GOG-Foundation Board of Directors, outside the submitted work.
Dr. Park received grant funding from NIH/NCI P30 CA008748.
Dr. Soslow is on a core grant from the NIH. He has received Royalties from Springer and Cambridge University Press and payment for development of educational presentations from Ebix/Oakstone.
All other co-authors have no Conflicts of Interest to declare.
NCT#: NCT00340808
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019., CA: A Cancer Journal for Clinicians. 69 (2019) 7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- [2].Eifel PJ, Ross J, Hendrickson M, Cox RS, Kempson R, Martinez A, Adenocarcinoma of the endometrium. Analysis of 256 cases with disease limited to the uterine corpus: treatment comparisons., Cancer. 52 (1983) 1026–1031. . [DOI] [PubMed] [Google Scholar]
- [3].Abeler VM, Kjorstad KE, Serous papillary carcinoma of the endometrium: a histopathological study of 22 cases., Gynecologic Oncology. 39 (1990) 266–271. 10.1016/0090-8258(90)90250-o. [DOI] [PubMed] [Google Scholar]
- [4].Aquino-Parsons C, Lim P, Wong F, Mildenberger M, Papillary serous and clear cell carcinoma limited to endometrial curettings in FIGO stage 1a and 1b endometrial adenocarcinoma: treatment implications., Gynecologic Oncology. 71 (1998) 83–86. 10.1006/gyno.1998.5147. [DOI] [PubMed] [Google Scholar]
- [5].Carcangiu ML, Chambers JT, Uterine papillary serous carcinoma: a study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma., Gynecologic Oncology. 47 (1992) 298–305. 10.1016/0090-8258(92)90130-b. [DOI] [PubMed] [Google Scholar]
- [6].Carcangiu ML, Tan LK, Chambers JT, Stage IA uterine serous carcinoma: a study of 13 cases., American Journal of Surgical Pathology. 21 (1997) 1507–1514. 10.1097/00000478-199712000-00015. [DOI] [PubMed] [Google Scholar]
- [7].Darvishian F, Hummer AJ, Thaler HT, Bhargava R, Linkov I, Asher M, Soslow RA, Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases., American Journal of Surgical Pathology. 28 (2004) 1568–1578. 10.1097/00000478-200412000-00004. [DOI] [PubMed] [Google Scholar]
- [8].Gitsch G, Friedlander ML, V Wain G, Hacker NF, Uterine papillary serous carcinoma. A clinical study., Cancer. 75 (1995) 2239–2243. . [DOI] [PubMed] [Google Scholar]
- [9].Nicklin JL, Copeland LJ, Endometrial papillary serous carcinoma: patterns of spread and treatment., Clinical Obstetrics and Gynecology. 39 (1996) 686–695. 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- [10].Altman AD, Ferguson SE, Atenafu EG, Köbel M, McAlpine JN, Panzarella T, Lau S, Gien LT, Gilks B, Clarke B, Cameron A, Nelson G, Han G, Samouëlian V, Ho TC, Louie K, Bernardini MQ, Canadian High Risk Endometrial Cancer (CHREC) consortium: analyzing the clinical behavior of high risk endometrial cancers., Gynecologic Oncology. 139 (2015) 268–274. 10.1016/j.ygyno.2015.09.001. [DOI] [PubMed] [Google Scholar]
- [11].Boruta DM 2nd, Gehrig PA, Groben PA, Bae-Jump V, Boggess JF, Fowler WCJ, Van Le L, Uterine serous and grade 3 endometrioid carcinomas: is there a survival difference?, Cancer. 101 (2004) 2214–2221. 10.1002/cncr.20645. [DOI] [PubMed] [Google Scholar]
- [12].Garg K, Leitao MMJ, Wynveen CA, Sica GL, Shia J, Shi W, Soslow RA, p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes., Modern Pathology. 23 (2010) 80–92. 10.1038/modpathol.2009.153. [DOI] [PubMed] [Google Scholar]
- [13].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA, Integrated genomic characterization of endometrial carcinoma., Nature. 497 (2013) 67–73. 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, Britton H, Kommoss F, Grevenkamp F, Karnezis A, Yang W, Lum A, Krämer B, Taran F, Staebler A, Lax S, Brucker SY, Huntsman DG, Gilks CB, McAlpine JN, Talhouk A, Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series., Annals of Oncology : Official Journal of the European Society for Medical Oncology. 29 (2018) 1180–1188. 10.1093/annonc/mdy058. [DOI] [PubMed] [Google Scholar]
- [15].Chen W, Husain A, Nelson GS, Rambau PF, Liu S, Lee C-H, Lee S, Duggan MA, Köbel M, Immunohistochemical Profiling of Endometrial Serous Carcinoma., International Journal of Gynecological Pathology : Official Journal of the International Society of Gynecological Pathologists. 36 (2017) 128–139. 10.1097/PGP.0000000000000291. [DOI] [PubMed] [Google Scholar]
- [16].Köbel M, Nelson GS, Letter in response to: McAlpine J, Leon-Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol 2018; 244: 538–549., The Journal of Pathology. 245 (2018) 249–250. 10.1002/path.5068. [DOI] [PubMed] [Google Scholar]
- [17].McAlpine J, Leon-Castillo A, Bosse T, The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses., The Journal of Pathology. 244 (2018) 538–549. 10.1002/path.5034. [DOI] [PubMed] [Google Scholar]
- [18].Crum CP, Lee KR, Diagnostic Gynecologic and Obstetric Pathology, Elsevier Saunders, Philadelphia, 2006. [Google Scholar]
- [19].Espinosa I, D’Angelo E, Corominas M, Gonzalez A, Prat J, Mixed endometrial carcinomas with a “low-grade serous”-like component: a clinicopathologic, immunohistochemical, and molecular genetic study., Human Pathology. 71 (2018) 65–73. 10.1016/j.humpath.2017.10.016. [DOI] [PubMed] [Google Scholar]
- [20].Öz M, Karalök A, Şirvan L, Taşçı T, Öcalan R, Turan AT, Güngör T, Meydanlı MM, Vanishing endometrial carcinoma in hysterectomy specimens: probable implications for fertility sparing management., Turkish Journal of Medical Sciences. 47 (2017) 1744–1750. 10.3906/sag-1607-93. [DOI] [PubMed] [Google Scholar]
- [21].Conlon N, Da Cruz Paula A, Ashley CW, Segura S, De Brot L, da Silva EM, Soslow RA, Weigelt B, DeLair DF, Endometrial Carcinomas with a “Serous” Component in Young Women Are Enriched for DNA Mismatch Repair Deficiency, Lynch Syndrome, and POLE Exonuclease Domain Mutations., American Journal of Surgical Pathology. 44 (2020) 641–648. 10.1097/PAS.0000000000001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].León-Castillo A, Gilvazquez E, Nout R, Smit VT, McAlpine JN, McConechy M, Kommoss S, Brucker SY, Carlson JW, Epstein E, Rau TT, Soslow RA, Ganesan R, Matias-Guiu X, Oliva E, Harrison BT, Church DN, Gilks CB, Bosse T, Clinicopathological and molecular characterisation of “multiple-classifier” endometrial carcinomas., The Journal of Pathology. 250 (2020) 312–322. 10.1002/path.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


