Clinical practice points
-
•
There is no real-world data in clinical practice for multiple myeloma in Lebanon
-
•
Despite novel therapies, high dose chemotherapy regimens are still used in the treatment of multiple myeloma.
-
•
Autologous stem cell transplant improves progression free survival and still a valid option for transplant fit patient despite novel therapeutic novel agents
-
•
OS and PFS were not affected by type of regimen
-
•
Hypercalcemia was significantly associated with a shorter mean PFS and OS upon presentation
-
•
Because most of the time real-world data patients will not meet eligibility criteria for clinical trials such as age and performance status, effort should be multiplied towards developing a Lebanese cancer data base (e.g clinical characteristics, survival outcomes of treatment, statistics…).
Keywords: transplant, induction protocol, progression free survival, overall survival, plasma cells disorder
Abstract
OBJECTIVE
The present retrospective multicenter study aims at documenting characteristics of multiple myeloma (MM) patients and the effect of autologous stem cell transplant (ASCT) on survival.
METHODS
A total of 134 adult patients initiating any new MM therapy from January 2002 till December 2019 were included. Enrollment was stratified by disease subtype, induction protocol and transplant status. The characteristics and survival outcomes were recorded.
RESULTS
Mean age at diagnosis was 61.91 ± 10.83 years, with 62.7% male patients. Regarding the prognostic MM International Staging System (ISS), stage 3 was the most common at diagnosis with 50.8% of patients followed by stage 1 (25.4%) and stage 2 (23.8%). Maintenance treatment was given in 88.5% of the patients. 24.6% patients were transplanted, 41% were not and the remaining were unknown or still in induction. 86.1% of patients were alive at data cut off. A significantly higher mean progression free survival (PFS) was found in transplant patients (p=0.016). Using cox regression, creatinine >2 mg/dl (HR3.78) and hypercalcemia >11 mg/dl (HR=6.48) were significantly associated with a shorter PFS1. A significantly shorter overall survival (OS) was associated with hypercalcemia (HR=6.58), as well as male gender though not statistically significant in the latter. Difference in survival distributions by treatment was not statistically significant (bortezomib thalidomide dexamethasone (VTD) (p=0.211), bortezomib cyclophosphamide dexamethasone (VCD) (p=0.111) or bortezomib Revlimid dexamethasone (VRD) (p=0.312)). The interaction between ISS stage on diagnosis and transplant was not significantly associated with the overall survival.
CONCLUSION
The results of our retrospective study are in conformity with international data emphasizing the role of transplant in the treatment algorithm of newly diagnosed transplant-eligible multiple myeloma patients.
Introduction
Multiple Myeloma (MM) a B lineage malignancy of plasmocytic cells predominating mainly in the bone marrow [1]. It is the second most common hematological malignancy, accounting for nearly 10% of all hematological malignant disorders and 0.9% of all cancer deaths every year [2]. MM incidence varies widely across countries but has known a steady increase since 1990 especially in low and middle sociodemographic index countries [3]. Incidence rate has increased by 126% globally and by between 106% to 192% according to sociodemographic index from 1990 to 20163.
Multiple myeloma is a spectrum of 4 major of disease entities with one progressing to another: the monoclonal gammopathy of unknown significance, the asymptomatic smoldering multiple myeloma, the so-called symptomatic myeloma, and finally plasma cell leukemia. These 3 diseases are characterized by an increase in clonal plasma cells in bone marrow as detected by an increase in monoclonal proteins in the blood or urine. Until very recently, the entity on the spectrum requiring therapy is symptomatic multiple myeloma which is associated with end organ damage. Plasma cell leukemia is a final, lethal form of the disease progression in which plasma cells circulate in the peripheral blood and for which no definitive effective approach is currently available [4]
Until very recently, symptomatic multiple myeloma disease, therefore the form requiring therapeutic intervention, was characterized by the “CRAB” criteria, defined as the presence of hypercalcemia, renal failure, anemia and bone disease [4]. With new data and advances, the “SLiM CRAB” criteria became the standard for defining symptomatic disease in order to start therapy. SLiM refer to a plasma cell count above 60% in the bone marrow, a ratio above 100 of involved over non-involved free Light chains and the presence of lytic lesion more than 5 mm on MRI [2].
The disease has also changed in regards to its prognosis and decision tools with the advent of genetic studies and FISH testing to determine high risk categories requiring more aggressive treatment approaches [5].
The backbone of treatment is currently a triplet protocol: a proteasome inhibitor with high dose steroids along with either an immunomodulator or chemotherapy (cyclophosphamide). In fit patients, the treatment protocol usually consists of an induction phase followed by autologous stem cell transplant, consolidation and maintenance therapies, respectively [6]. New protocols are recently being investigated in the induction phase such as new generation of proteasome inhibitors (Carfilzomib) or monoclonal antibodies (Daratumumab) in order to improve the prognosis of patients especially in terms of progression-free survival (PFS) and overall survival (OS).
Autologous stem cell transplant (ASCT) remains a must in all used approaches, with recent data and protocols having not succeeded in removing transplant from the treatment strategy [7]. Moreover, high risk patients might need a double tandem transplant [7]. Early ASCT is the standard of care, improving outcomes regardless of whether it is performed in the frontline setting or as salvage.
As of yet, MM is considered a treatable but incurable disease, and thus, lifelong observation and follow-up are recommended. The International staging system (ISS) for Multiple Myeloma is widely used for prognostic staging and has recently been revised. The ISS stratifies patients into 3 stages (I, II, and III) according to serum ß-2 Microglobulin and serum albumin levels, with different median survival values for each stage. The ISS and renal failure significantly affect overall survival in South Asian populations but hypercalcemia, anemia and bone involvement impact were statistically non significant [8,9]. Transplantation was also associated with a significantly increased complete response. High-dose therapy combined with transplantation improves the response rate, event free survival, and overall survival in patients with myeloma [10].
The clinical characteristics of newly diagnosed multiple myeloma and their treatment modalities in Lebanon have not yet been well defined nor reported. Therefore, more reporting of real world data of multiple myeloma in Lebanon will lead to an increase in disease understanding which may improve patient care. The objective of this retrospective study is to describe the clinical and laboratory features of multiple myeloma patients at diagnosis in Lebanon and investigate prognostic factors and treatment protocols that correlate with survival outcomes in the Lebanese population.
Methods
Study design
The medical records were retrospectively reviewed of Multiple Myeloma patients between January 2002 and December 2019. Data was collected from 134 patients’ medical files of Notre Dame de Secours University Hospital and Mount Lebanon Hospital. Inclusion criteria comprised patients newly diagnosed with Multiple Myeloma and receiving triplet combination treatment. All patients included in the study have data on all sequences of therapy. For transplant patients, ASCT was realized after induction therapy (early). Diagnosis of Multiple Myeloma was made according to international guidelines. Patients diagnosed with Smoldering Multiple Myeloma were not included in the study. The last data update of the data was done in February 2020.
Ethical Approval
The Institutional Review Board of both hospitals approved the study protocol based on the fact that it is a retrospective observational study conferring a respect to patient's autonomy and confidentiality and caused minimal harm.
Sample Size Calculation
Using the Epi-info software and based on the frequency of 1.5% of MM in the absence of studies in Lebanon, with an acceptable margin of error of 5% and a design effect of 2, a minimal sample size of 46 patients was deemed necessary. We collected 134 patients after excluding all missing files (40) from our registry.
Data Entry
Collected characteristics included patient's age at diagnosis, sex, disease subtype (IgM, IgA, IgG, IgD, κ, λ, IgGλ, IgGκ, IgAκ, IgMλ, IgGκ + IgMλ, IgMκ), diagnostic criteria (bone marrow biopsy, SPEP, UPEP, Serum IF, Urine IF, Unknown), percentage of clonal plasmocytes in bone marrow, presence of anemia (Hb<10g/dL), renal function (Creatinine >2mg/dL or creatinine clearance <40mL/min), hypercalcemia (>1mg/dL higher than the upper limit of normal or >11 gm/dL), presence of lytic lesions, cytogenetic (normal, not normal), ISS score, induction protocol (VTD, VRD, VED, VER, VD, D, oncovin, Thalidomide, Endoxan, RD, MPD, Radiotherapy, VAdriaD+T, HyperCVAD, VDT+Alkeran, MP or V+Caelyx+D), transplant status, use of consolidation, use of maintenance, and progression criteria along with survival data analysis. For those who did not receive consolidation, either six cycles of induction were done before transplant, or shifted to maintenance directly due to toxicity, or patient couldn't afford prices of treatment to continue. Regarding cytogenetics, Karyotype was previously done on all cells, later on CD38+ plasma cells which is only available at a single lab which could explain the high number of normal Karyotype (FISH technique is highly expensive in Lebanon and cannot be affordable to all patients). Progression free survival was defined as the duration of time from first day of starting therapy until the day of progression of any line of treatment. Overall survival (OS) was defined as the duration of time from diagnosis to death regardless of the cause of death.
Statistical Analysis
Data was analyzed using the SPSS software version 25. Log-rank test and Cox regression analysis were applied to identify predictors of mortality, using the proportional hazards assumption. Observed PFS and OS were computed for transplant status and the type of induction protocol. Curves were calculated using the Kaplan-Meyer technique. For all statistical tests, a p<0.05 was considered statistically significant.
Results
Sociodemographic characteristics
Mean age of diagnosis was 61.91 ± 10.83 years. 62.7% were males and 37.3% were females. Regarding the M protein component, we had 52.2% IgG, 17.9% IgA, 1.4% IgM, 11.2% Kappa, 5.2% lambda, and 0.7% had 2 spikes M and G. 33% of the patients had anemia at diagnosis, 15.6% had elevated levels of creatinine and 8.7% presented hypercalcemia at the time of diagnosis. Regarding the prognostic MM International Staging System (ISS), stage 3 was the most common at diagnosis with 50.8% of patients followed by stage 1 (25.4%) and stage 2 (23.8%). VCD was the most frequently used induction protocol (38.8%) followed by VTD (14.2%) and VRD (9%). No deaths occurred during induction. 33 (37.5%) patients underwent autologous stem cell transplantation (ASCT) whereas 55 (62.5%) did not undergo transplant during any stage of treatment. The average age at transplant was 55.98 years ± 9.88 with 67.9% of these patients being males. Consolidation was given to 35.9% of the patients. 88.5% received a maintenance treatment. At the time of data analysis, 99 (86.1%) patients were still alive. An average overall survival of 53.57 months was found. During the study conduct, 55% and 29% of patients received second and third line of treatment respectively. Results are summarized in table 1.
Table 1.
Sociodemographic and other characteristics of the patients
| Variable | N (%) |
|---|---|
| Gender | |
| Males | 84 (62.7%) |
| Females | 50 (37.3%) |
| Diagnostic criteria: bone marrow (yes) | 9 (9.0%) |
| Diagnostic criteria: EPP (yes vs no*) | 30 (30.0%) |
| Diagnostic criteria: IE (yes vs no*) | 59 (59.0%) |
| Plasmocytes | |
| <10 | 34 (40.0%) |
| 10-60 | 39 (45.9%) |
| >60 | 12 (14.1%) |
| Caryotype | |
| Normal | 30 (78.9%) |
| Abnormal | 8 (21.1%) |
| Anemia (yes) | 37 (33.0%) |
| Renal disease (yes) | 17 (15.6%) |
| Calcemia (yes) | 9 (8.7%) |
| Presence of lytic lesions (yes) | 71 (75.5%) |
| B2 microglobulin level | |
| <3.5 | 18 (27.3%) |
| 3.5-5.3 | 19 (28.8%) |
| ≥5.4 | 29 (43.9%) |
| Albumin level | |
| ≥3.5 | 53 (61.6%) |
| <3.5 | 33 (38.4%) |
| Staging | |
| Stage 1 | 16 (25.4%) |
| Stage 2 | 15 (23.8%) |
| Stage 3 | 32 (50.8%) |
| Transplant | |
| No | 55 (62.5%) |
| Yes | 33 (37.5%) |
| 2nd line tratment | 37 (55%) |
| 3rd line tratment | 11 (29%) |
| Consolidation treatment (yes) | 23 (35.9%) |
| Maintenance (yes) | 54 (88.5%) |
| Progression (yes) | 52 (46.4%) |
| Death | |
| No | 99 (86.1%) |
| Yes | 16 (13.9%) |
| Mean ± SD | |
| Age (in years) | 61.91 ± 10.83 |
| Overall survival (in months) | 53.57 ± 45.98 |
Cox regression
Taking PFS1 as the dependent variable, the results of the Cox regression showed that renal failure evaluated by a creatinine >2mg/dL (p=0.037) and hypercalcemia >11 mg/dL (p=0.008) were significantly associated with a shorter time to first progression (Table 2). A shorter overall survival was significantly associated with having hypercalcemia upon presentation (HR=6.58, p=0.006). Male gender (HR=0.30, 0=0.078) was associated with a shorter overall survival but this association was not statistically significant (Table 3).
Table 2.
Explanatory variables associated with progression free survival. Multivariable analysis with cox regression taking the PFS1 as the dependant variable
| Model 1: Cox regression taking the PFS1 as the dependent variable. | ||||
|---|---|---|---|---|
| Variable | Unadjusted HR | p; 95% CI | Adjusted HR | p; 95% CI |
| Age | 1.02 | 0.361; 0.98-1.06 | ||
| Gender (females vs males*) | 0.30 | 0.065; 0.09-1.08 | ||
| Diagnostic criteria: bone marrow (yes vs no*) | 1.44 | 0.665; 0.28-7.37 | ||
| Diagnostic criteria: EPP (yes vs no*) | 0.76 | 0.672; 0.21-2.78 | ||
| Diagnostic criteria: IE (yes vs no*) | 1.20 | 0.763; 0.36-4.02 | ||
| Plasmocytes | ||||
| <10 | 1 | |||
| 10-60 | 5.40 | 0.119; 0.65-44.90 | ||
| >60 | 3.29 | 0.400; 0.21-52.64 | ||
| Caryotype (abnormal vs normal*) | 2.81 | 0.305; 0.39-20.10 | ||
| Anemia (yes vs no*) | 2.05 | 0.171; 0.74-5.70 | ||
| Renal disease (yes vs no*) | 3.78 | 0.037; 1.08-13.20 | 2.59 | 0.230; 0.55-12.30 |
| Calcemia (yes vs no*) | 6.48 | 0.008; 1.63-25.76 | 3.71 | 0.122; 0.70-19.58 |
| Presence of lytic lesions (yes vs no*) | 1.01 | 0.982; 0.31-3.31 | ||
| B2 microglobulin | ||||
| <3.5 | 1 | |||
| 3.5-5.3 | 0.57 | 0.643; 0.05-6.26 | ||
| ≥5.4 | 0.74 | 0.745; 0.12-4.48 | ||
| Albumin (≥3.5 vs <3.5*) | 2.30 | 0.239; 0.58-9.21 | ||
| Staging | ||||
| Stage 1 | 1 | |||
| Stage 2 | 1.16 | 0.917; 0.07-18.56 | ||
| Stage 3 | 1.54 | 0.699; 0.17-13.93 | ||
| Transplant (yes vs no*) | 1.42 | 0.595; 0.39-5.19 | ||
| Consolidation treatment (yes vs no*) | 0.73 | 0.693; 0.16-3.45 | ||
| Progression (yes vs no*) | 1.14 | 0.795; 0.42-3.09 | ||
Numbers in bold indicate significant p-values.
Table 3.
Explanatory variables associated with progression free survival. Multivariable analysis with cox regression taking the PFS1 as the dependant variable. Variables entered Variables entered in the final model: sex, calcemia, renal disease, anemia, plasmocytes
| Model 2: Cox regression taking the overall survival (in months) as the dependent variable. | ||||
|---|---|---|---|---|
| Variable | Unadjusted HR | p; 95% CI | Adjusted HR | p; 95% CI |
| Age | 1.02 | 0.410; 0.98-1.06 | ||
| Gender (females vs males*) | 0.32 | 0.078; 0.09-1.13 | ||
| Diagnostic criteria: bone marrow (yes vs no*) | 1.90 | 0.409; 0.41-8.72 | ||
| Diagnostic criteria: EPP (yes vs no*) | 0.98 | 0.983; 0.27-3.60 | ||
| Diagnostic criteria: IE (yes vs no*) | 0.83 | 0.748; 0.27-2.55 | ||
| Plasmocytes | 0.129 | |||
| <10 | 1 | |||
| 10-60 | 7.95 | 0.053; 0.97-65.07 | ||
| >60 | 3.18 | 0.415; 0.20-51.18 | ||
| Caryotype (abnormal vs normal*) | 2.52 | 0.358; 0.35-17.95 | ||
| Anemia (yes vs no*) | 2.00 | 0.182; 0.72-5.56 | ||
| Renal disease (yes vs no*) | 2.53 | 0.117; 0.79-8.09 | ||
| Calcemia (yes vs no*) | 6.58 | 0.006; 1.70-25.48 | 11.39 | 0.002; 2.45-52.88 |
| Presence of lytic lesions (yes vs no*) | 0.73 | 0.59; 0.23-2.33 | ||
| B2 microglobulin | 0.784 | |||
| <3.5 | 1 | |||
| 3.5-5.3 | 0.48 | 0.546; 0.04-5.30 | ||
| ≥5.4 | 0.58 | 0.558; 0.09-3.62 | ||
| Albumin (≥3.5 vs <3.5*) | 2.28 | 0.220; 0.61-8.53 | ||
| Staging | 0.939 | |||
| Stage 1 | 1 | |||
| Stage 2 | 0.95 | 0.968; 0.06-15.34 | ||
| Stage 3 | 1.32 | 0.804; 0.14-12.21 | ||
| Transplant (yes vs no*) | 1.07 | 0.915; 0.30-3.86 | ||
| Consolidation treatment (yes vs no*) | 0.76 | 0.730; 0.16-3.68 | ||
| Progression (yes vs no*) | 0.63 | 0.370; 0.23-1.73 | ||
Numbers in bold indicate significant p-values
Survival analysis
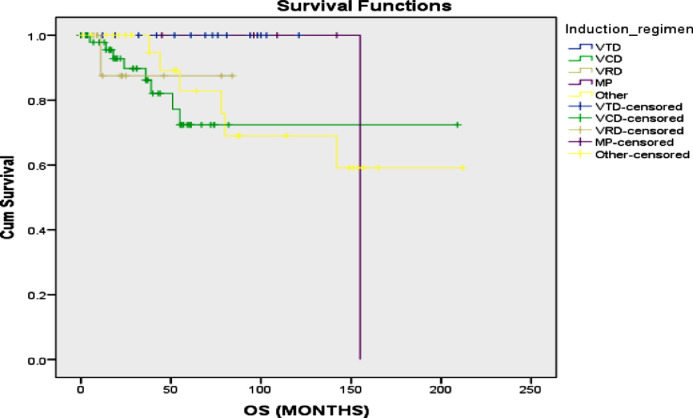
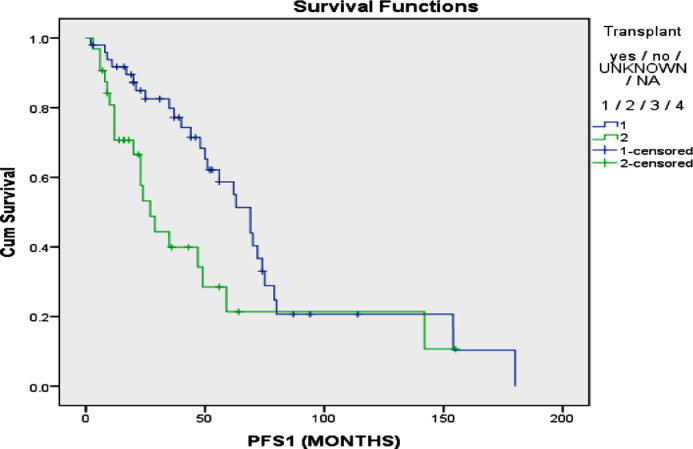
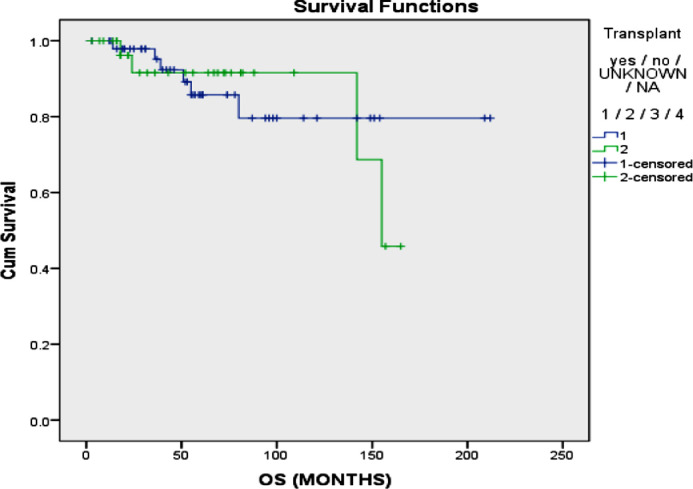
No significant difference was found in terms of mean PFS between those who received VTD (48.6 vs 75.60; p=0.211), VCD (63.91 vs 75.24; p=0.111) or VRD (70.09 vs 69.07; p=0.312) as an induction treatment (figure 1). Additionally, no significant difference was found in terms of OS between those who received VCD (160.98 vs 167.52; p=0.107 or VRd (74.87 vs 159.60; p=0.855) orVTd (155.00 vs 162.79; p=0.614) as an induction treatment (figure 2). A significantly higher mean progression free survival (PFS) was found in patients who received a transplant compared to those who did not (75.11 vs 51.98 months; p=0.016) (figure 3). However, no significant difference in terms of mean OS was found in patients who received a transplant compared to those who did not (179.56 vs 145.33 months; p=0.915) (figure 4). The interaction between ISS stage on diagnosis and transplant was not significantly associated with the mortality or overall survival.
Figure 1.
Progression free survival of multiple myeloma with different chemotherapy regimens
Figure 2.
Overall survival of multiple myeloma patients treated patients treated with different chemotherapy regimens
Figure 3.
Progression free survival of multiple myeloma in patients patients who underwent transplant vs. those who did not
Figure 4.
Overall survival of multiple myeloma in who underwent transplant vs. those who did not.
Discussion
In our population, patients presented from different regions across Lebanon and were not confined to a single geographical area. This is helpful for further inferences concerning the general population. Compared to international data [11], a slightly lower median age at diagnosis was found in our study, probably due to an earlier presentation or detection of the disease. Additionally, we found that men were 1.7 times more affected than women, and the most predominant disease subtype was IgG followed by IgA. These results were also in concordance with those found in international data [11]. The majority of our patients presented with ISS stage III disease. These results might raise questions concerning the aggressiveness of the disease in our population. This could be related to cytogenetics and further studies will be needed concerning this subject. In terms of transplant, the age range paralleled that of international guidelines [12].
However, 17 patients in the non-transplant group with an age range of 41 to 67 years did not receive a transplant. This could be attributed to logistical issues, psychosocial factors, and lack financial resources especially considering that ASCT in Lebanon is not always covered by third-party payment providers. Additionally, misconceptions about safety and mortality concerning ASCT are major issues interfering in the decision-making process. We found a significant difference in mean PFS between the transplanted and the non-transplanted group, in a way that ASCT was associated with a higher mean PFS. This result was in conformity with international study that proved the effectiveness of ASCT in lengthening the time to first progression [7]. In fact, delaying first progression in transplant patients might have an important psychological impact and improves quality of life [13]. Patients will be more comfortable after ASCT and willing to continue pursue their treatment. However, more studies are needed concerning this field. On the other hand, we found no difference in mean OS, whether MM patients were transplanted or not. When confronted with other studies, mixed results concerning OS were found. While some proved a significant difference in terms of OS [14], [15], [16]. Others did not show a difference between the OS of transplanted and non-transplanted patients [17,18]. First of all, the elevated values of OS obtained in our study were similar to another retrospective analysis comparing transplant and non-transplant groups [18]. This could be assigned to the use of novel agents (proteasome inhibitors and immunomodulators) [17]. The lack of OS benefit in ASCT found in our study could primarily be due to our small sample size. Using the cox regression results, hypercalcemia >11 mg/dl was significantly associated with a shorter mean PFS and OS upon presentation. In reference to the Durie-Salomon staging system, elevated levels of calcium are associated with a higher tumor burden, hence worse prognosis [19]. As it was recently reported, the presence of hypercalcemia is an indicator of advanced disease and was associated with low performance status, anemia, renal failure, thrombocytopenia, elevated ISS along with a significant number of bone lesions [20]. Additionally, the presence of hypercalcemia was associated with high-risk cytogenetics, which would raise questions concerning the risk stratification of patients [21]. Early death was also reported in patients presenting with elevated of calcium as the risk increased by twofold compared to other patients [21]. Renal failure defined as a creatinine >2 mg/dL showed an association with a shorter PFS which was consistent with international data. Elevated creatinine levels are also associated with advanced disease and patients may present lower response to chemotherapy compared to patients without normal levels. However, with the use of novel agents, renal failure can be overcome. Patients with a creatinine < 4 mg/dL exhibited a reversible renal function resulting in better survival outcomes in comparison with patients having irreversible renal damage [22]. According to Khan et al., despite bortezomib-based treatment that can be used in patients with renal insufficiency, PFS was significantly altered for patients having a GFR <30 mL/min/1.73m2 [23]
Male gender (HR=0.32) was associated with a shorter overall survival but this association was not statistically significant. The results of myeloma XI trial showed no difference in survival outcomes between gender [24]
In terms of induction therapy the most used regimen was VCD. No significant difference was found in terms of mean PFS and OS among those who received VTD, VCD or VRD as an induction treatment. These results paralleled those of other studies stating that no major difference was described between the main regimens. However, it was recently reported that triplet induction therapy VRD was superior as regards to response rates [25]. Thus, to our knowledge, no gold standard treatment exists in terms of induction therapy. The choice of regimen depends on the availability, contraindications and experience of the clinician with the drug.
Limitations
Our major limitation concerning this study was the sample size. Several files had to be omitted for issues of missing data predisposing us to a selection bias, in addition to the collection of data from two hospitals only. A residual confounding bias is also possible since not all factors associated with PFS and OS were taken into account in this paper. More specifically, additional information concerning transplant, response rate well as relapse rate need to be reported for matters of completion and better inferences. Retrospective studies may show some limitations as disease subtypes and treatment history might bias results. Personality and psychosocial aspects might affect the choice of transplant too and these were not taken into consideration as it was a retrospective study. Moreover, patients receiving transplant are younger than those who don't thus having less comorbidities and better ability to tolerate somewhat intensive therapy. Therefore, better outcomes when it comes to survival [26]. Finally, the subject of ASCT is far from being fully elucidated. Heterogeneity between patients and prior history of treatment before transplant are important weaknesses that should be addressed.
Conclusion
In regard to our results, multiple myeloma patients have a median of 61 years of age and the majority present with advanced stage. A minority of patients received more than two lines of therapy and profited from the prescribed treatment regimens. The results of our retrospective study are in conformity with international data emphasizing the role of transplant in the treatment algorithm of newly diagnosed transplant-eligible multiple myeloma patients. The role of transplant remains undebatable as of yet, no new therapy has resulted in avoidance of this approach.
To our knowledge, this study is a first real world data report of multiple myeloma in Lebanon. Finally, this view into patient population in clinical world practice can be beneficial as it gives rise to broader understanding of the Lebanese patient journeys and of the level of treatment efficacy, hence revealing the category of patients with increased benefit or risk
Funding source
This research did not receive any specific grant from funding agencies in the public commercial or not-for-profit sectors
CRediT authorship contribution statement
Fadi Nasr: Methodology, Supervision, Conceptualization, Validation, Writing - review & editing, Visualization. Ahmad Al Ghoche: Conceptualization, Investigation, Visualization, Writing - review & editing. Saada Diab: Conceptualization, Investigation, Writing – original draft, Visualization. Lewis Nasr: Investigation, Writing - review & editing, Visualization. Emmanuel Ammanouil: Writing - review & editing, Visualization. Christelle Riachy: Writing - review & editing, Visualization. Souheil Hallit: Formal analysis. Georges Chahine: Validation, Writing - review & editing, Visualization.
References
- 1.Klein B. Survival and proliferation factors of normal and malignant plasma cells. Int. J. Hematol. 2003;78:106–113. doi: 10.1007/BF02983377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar S.V. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 3.Cowan A.J. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2016;4:1221–1227. doi: 10.1001/jamaoncol.2018.2128. (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talamo G. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10:464–468. doi: 10.3816/CLML.2010.n.080. [DOI] [PubMed] [Google Scholar]
- 5.Morrison T., Booth R.A., Hauff K., Berardi P., Visram A. Laboratory assessment of multiple myeloma. Adv Clin Chem. 2019;89:1–58. doi: 10.1016/bs.acc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Child J.A. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 7.Hamed Al, R. Bazarbachi, H. A., Malard F., Harousseau J.-L., Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9 doi: 10.1038/s41408-019-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hameed A. Characteristics and outcomes of patients with multiple myeloma : Data from a developing country. Med J Islam Repub Iran. 2018;32:1. doi: 10.14196/mjiri.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basit A., Siddiqui N., Hameed A., Muzaffar N., Athar S. FACTORS AFFECTING OUTCOME OF PATIENTS WITH MULTIPLE MYELOMA. Journal of Ayub Medical College Abbottabad. 2014;26:376–379. [PubMed] [Google Scholar]
- 10.Attal M. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N. Engl. J. Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 11.Kyle R.A. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar S.V. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. American Journal of Hematology. 2018;93:1091–1110. doi: 10.1002/ajh.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laubach J., Kumar S. Management of Transplant-Eligible Patients with Newly Diagnosed Multiple Myeloma. Cancer Treat. Res. 2016;169:145–167. doi: 10.1007/978-3-319-40320-5_9. [DOI] [PubMed] [Google Scholar]
- 14.Gay F. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–1629. doi: 10.1016/S1470-2045(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. New England Journal of Medicine. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 16.Attal M. A Prospective, Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma. New England Journal of Medicine. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 17.Attal M. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. New England Journal of Medicine. 2017;376:1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal M. Autologous stem cell transplantation in first remission is associated with better progression-free survival in multiple myeloma. Ann. Hematol. 2018;97:1869–1877. doi: 10.1007/s00277-018-3370-1. [DOI] [PubMed] [Google Scholar]
- 19.Durie-Salmon Staging System. International Myeloma Foundation https://www.myeloma.org/durie-salmon-staging.
- 20.Kastritis E. Frequency and Prognostic Significance of Hypercalcemia in Patients with Multiple Myeloma: An Analysis of the Database of the Greek Myeloma Study Group. Blood. 2011;118 5083–5083. [Google Scholar]
- 21.Zagouri F. Hypercalcemia remains an adverse prognostic factor for newly diagnosed multiple myeloma patients in the era of novel antimyeloma therapies. Eur. J. Haematol. 2017;99:409–414. doi: 10.1111/ejh.12923. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen L.M., Hjorth M., Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. European Journal of Haematology. 2000;65:175–181. doi: 10.1034/j.1600-0609.2000.90221.x. [DOI] [PubMed] [Google Scholar]
- 23.Khan R. Renal insufficiency retains adverse prognostic implications despite renal function improvement following Total Therapy for newly diagnosed multiple myeloma. Leukemia. 2015;29:1195–1201. doi: 10.1038/leu.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bird S.A. Sex Differences in Multiple Myeloma Biology and Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Blood. 2019;134 doi: 10.1016/j.clml.2021.04.013. 4374–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar L., Chellapuram S., kumar, Sahoo R., Gupta R. VRd versus VCd as induction therapy for newly diagnosed multiple myeloma: A Phase III, randomized study. Clinical Lymphoma, Myeloma and Leukemia. 2019;19:e361. [Google Scholar]
- 26.Rajkumar S.V., Sonneveld P. Front-Line Treatment in Younger Patients With Multiple Myeloma. Semin Hematol. 2009;46:118–126. doi: 10.1053/j.seminhematol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]