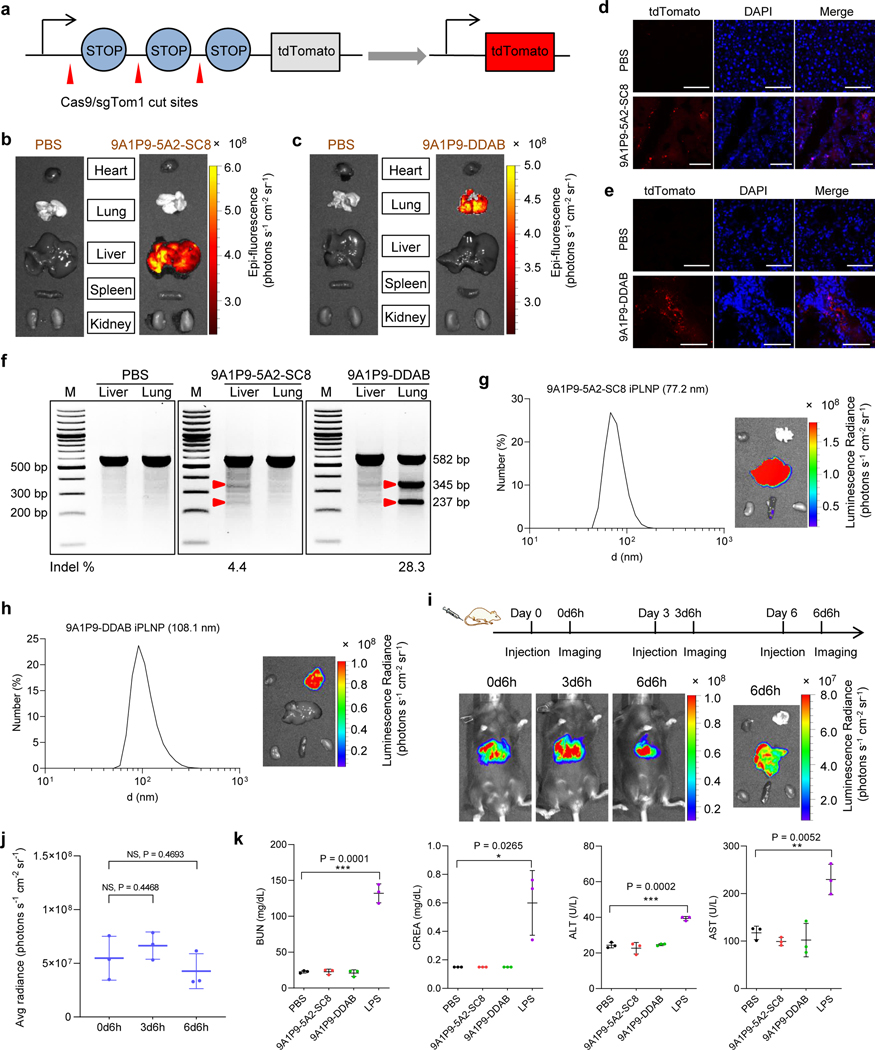
Fig. 6 ǀ. iPLNPs enabled CRISPR/Cas9 gene editing selectively in liver and lungs and possessed potential for clinical translation.
a, Schematic of co-delivery of Cas9 mRNA and sgTom1 deletes the stop cassettes and activates tdTomato protein. b, 9A1P9–5A2-SC8 iPLNPs enabled gene editing specifically in liver. c, 9A1P9-DDAB iPLNPs enabled gene editing specifically in lung. d, Following IV administration of 9A1P9–5A2-SC8 iPLNPs containing Cas9 mRNA and sgTom1 to Ai9 mice, tdTomato-positive cells were observed in liver. Scale bars, 50 μm. e, Confocal fluorescence images showed tdTomato-positive cells in lung after administration of 9A1P9-DDAB iPLNPs. Scale bars, 50 μm. f, T7E1 assay of organ selective gene editing. 9A1P9–5A2-SC8 and 9A1P9-DDAB iPLNPs containing Cas9 mRNA and sgPTEN were IV administered into C57BL/6 mice, enabling CRISPR/Cas9 gene editing in liver and lung, respectively. For all the CRISPR/Cas9 gene editing assays, Cas9 mRNA/sgRNA weight ratio of 4:1 and total RNA dose of 0.75 mg kg−1 were used. g,h, iPLNPs were prepared by controlled microfluidic mixing, which resulted in decreased iPLNP sizes and preserved efficacy and organ selectivity. 9A1P9–5A2-SC8 iPLNPs (liver specific, Fluc mRNA, 0.05 mg kg−1) (g) and 9A1P9-DDAB iPLNPs (lung specific, Fluc mRNA, 0.25 mg kg−1) (h) demonstrated small sizes and fully retained precise organ selectivity. i,j, 9A1P9–5A2-SC8 iPLNPs (Fluc mRNA, 0.05 mg kg−1) allowed repeat dosing without loss of efficacy. Whole body imaging (i) and quantification of luciferase expression (j) was performed 6 h after each injection. k, 9A1P9–5A2-SC8 and 9A1P9-DDAB iPLNPs were well tolerated in vivo. Data are presented as mean ± s.d. and statistical significance was analyzed by the two-tailed unpaired t-test: ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05; NS, P > 0.05. All data are from n = 3 biologically independent mice.