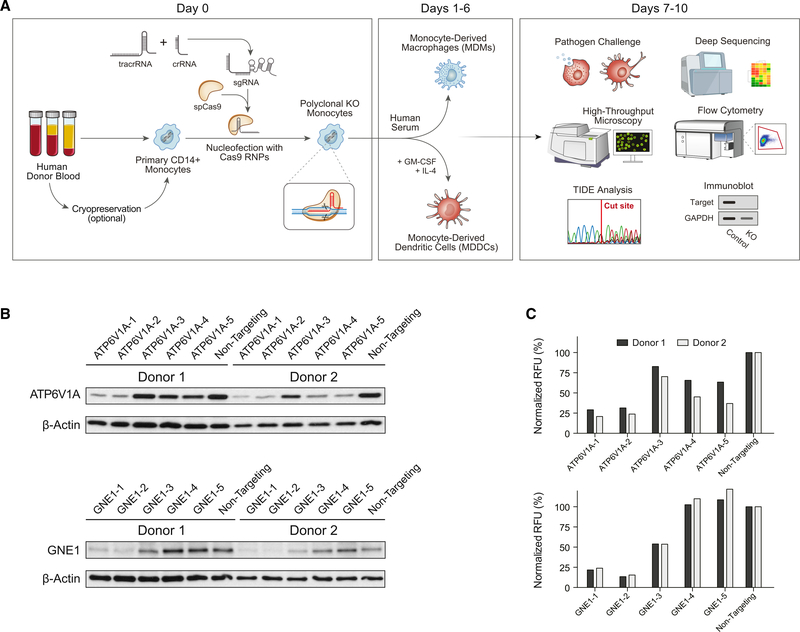
Figure 1. A flexible platform for CRISPR editing of human myeloid-lineage cells.
(A) A generalized schematic of the platform. Human CD14+ monocytes are isolated from blood by density gradient separation of PBMCs followed by magnetic negative selection. Either PBMCs or monocytes may be cryopreserved for later editing (Figure S1B). Cells are then nucleofected with preformed CRISPR-Cas9 RNPs and immediately put into differentiating culture under MDM- or MDDC-generating conditions. After allowing for 6–7 days of differentiation and washout of the targeted gene product, cells can be subjected to a wide variety of functional, phenotypic, and genotypic studies to assess the knockout efficiency and function of the targeted gene product.
(B) Guide sequence-dependent knockout of targeted genes leads to loss of gene products. CD14+ monocytes were nucleofected with RNPs containing 1 of 5 distinct guide sequences against the indicated gene or a scrambled non-targeting control, cultured under MDM-generating conditions, and then lysed for immunoblot analysis. Blots show targeted gene protein product and untargeted housekeeping gene product β-actin protein levels in cells from 2 blood donors. GNE1 and ATP6V1A ran at their expected sizes of 79 and 69 kDa, respectively.
(C) Knockout was quantified by digital densitometry and normalized on a per-sample basis in relative fluorescence units (RFUs) to untargeted housekeeping control protein β-actin.
See also Figure S1.