Abstract
Introduction
Commercially available serological assays for SARS-CoV-2 detect antibodies to either the nucleocapsid or spike protein. Here we compare the performance of the Beckman-Coulter SARS-CoV-2 spike IgG assay to that of the Abbott SARS-CoV-2 nucleocapsid IgG and Roche Anti-SARS-CoV-2 nucleocapsid total antibody assays. In addition, we document the trend in nucleocapsid and spike antibodies in sequential samples collected from convalescent plasma donors.
Methods
Plasma or serum samples from 20 individual SARS-CoV-2 RT-PCR-positive inpatients (n = 172), 20 individual convalescent donors with a previous RT-PCR-confirmed SARS-CoV-2 infection (n = 20), were deemed positive SARS-CoV-2 samples. RT-PCR-negative inpatients (n = 24), and 109 pre-SARS-CoV-2 samples were determined to be SARS-CoV-2 negative. Samples were assayed by the Abbott, Roche, and Beckman assays.
Results
All three assays demonstrated 100% specificity. Abbott, Beckman, and Roche platforms had sensitivities of 98%, 93%, and 90% respectively, with the difference in sensitivity attributed primarily to samples from immunocompromised patients. After the exclusion of samples immunocompromised patients, all assays exhibited ≥ 95% sensitivity. In sequential samples collected from the same individuals, the Roche nucleocapsid antibody assay demonstrated continually increasing signal intensity, with maximal values observed at the last time point examined. In contrast, the Beckman spike IgG antibody signal peaked between 14 and 28 days post positive SARS-CoV-2 PCR and steadily declined in subsequent samples. Subsequent collections 51–200 days (median of 139 days) post positive SARS-CoV-2 RT-PCR from five inpatients and five convalescent donors revealed that spike and nucleocapsid antibodies remained detectable for several months after confirmed infection.
Conclusions
The three assays are sensitive and specific for SARS-CoV-2 antibodies. Nucleocapsid and spike antibodies were detectable for up to 200 days post-positive SARS-CoV-2 PCR but demonstrated markedly different trends in signal intensity.
Abbreviations: RT-PCR, reverse transcriptase polymerase chain reaction; COI, cutoff index; S/CO, signal to calibrator ratio
Keywords: COVID-19, SARS-CoV-2, Antibody test
1. Introduction
The diagnostic landscape for SARS-CoV-2 has changed depending on testing availability, treatment options, and our understanding of the virus. Diagnostic testing for SARS-CoV-2 is performed via reverse-transcriptase PCR (RT-PCR) [1]. However, RT-PCR testing detects current SARS-CoV-2 infections, but not previous infections or immunity. Serological testing fills this niche, with seroconversion generally 7–14 days after infection [2]. With the recent availability of vaccines to SARS-CoV-2 [3] testing for antibody status may become invaluable for assessment of immunity and epidemiology.
Because there is no consensus concerning which SARS-CoV-2 antibody may confer lasting immunity, it is important to assess performance of various antibody assays. The anti-SARS-CoV-2 assays available in the United States through the FDA’s emergency use authorization detect IgG, IgA, IgM, or total antibodies against SARS-CoV2 nucleocapsid or spike protein, with both strategies demonstrating advantages [4]. The nucleocapsid protein is immunogenic and highly conserved, making it theoretically less susceptible to genetic variation [5], [6]. In contrast, the spike protein is the target for neutralizing antibodies [7]. While antibodies to both viral proteins are observed following SARS-CoV-2 infection, more studies are needed to determine the antibody longevity.
Previously, we evaluated the Abbott SARS-CoV-2 IgG and Roche Elecsys Anti-SARS-CoV-2 total antibody assays, both which target the nucleocapsid protein [8]. To supplement our previous report, we have compared the performance of the Beckman-Coulter Access IgG spike protein assay to our previous report of Abbott and Roche assays. We also examine the persistence of antibody response to SARS-CoV-2 in convalescent donors and hospitalized patients with confirmed SARS-CoV-2 infection, over several months.
2. Methods
2.1. Sample collection
Plasma or serum samples from 20 inpatients (n = 172) positive for SARS-CoV-2 infection (via RT-PCR) as well as 20 convalescent donors (n = 20) with documented positive SARS-CoV-2 RT-PCR result were collected as SARS-CoV-2 positive samples. Samples from positive inpatients and convalescent donors were collected 0–35 days and 32–54 days post positive RT-PCR confirmation, respectively. Only one sample per time point per patient was included. 24 plasma/serum samples from 24 unique inpatients who tested negative for SARS-CoV-2 within one day of collection, and 105 remnant pre-SARS-CoV-2 samples (collected/stored between September2017 and June 2019 at −20 °C) were used as SARS-CoV-2 negative samples.
2.2. Longitudinal study
In a separate longitudinal study, 13 additional samples from five positive inpatients and five convalescent donors were collected and tested with the Roche and Beckman assays. The time between first positive SARS-CoV-2 RT-PCR result and sample collection ranged from 138 to 200 days for convalescent plasma donors, and 31–123 days for inpatients. These samples were not included in sensitivity/specificity calculations (Table 1 ).
Table 1.
Performance characteristics of Abbott, Beckman, and Roche SARS-CoV-2 serology assays.
| SARS-CoV-2 RT-PCR Result | Days since positive SARS-CoV-2 RT-PCR Result | Abbott (%) | Beckman (%) | Roche (%) |
|---|---|---|---|---|
| Positive | 0–6 | 43/45 (96%) | 35/45 (78%) | 37/45 (82%) |
| 7–13 | 61/61 (100%) | 60/61 (98%) | 61/61 (100%) | |
| ≥14* | 85/86 (99%) | 84/86 (98%) | 75/86 (87%) | |
| Total | 189/192 (98%) | 179/192 (93%) | 173/192 (90%) | |
| Total Excluding Immunosuppressed Patients (n = 2) | 174/177 (98%) | 169/177 (95%) | 173/177 (98%) | |
| Negative | Inpatient | 24/24 (100%) | 24/24 (100%) | 24/24 (100%) |
| Pre-Covid | 105/105 (100%) | 105/105 (100%) | 105/105 (100%) | |
| *Convalescent donor samples were included in ≥ 14 days calculations. | ||||
2.3. Data acquisition
All samples were analyzed with the Roche Anti-SARS-CoV-2 total antibody assay, Abbott SARS-CoV-2 IgG assay, and the Beckman-Coulter SARS-CoV-2 IgG assay. The Roche assay detects anti-nucleocapsid IgG, IgM, and IgA antibodies, the Abbott assay detects anti-nucleocapsid IgG antibodies, and the Beckman assay detects anti-spike IgG antibodies (see Supplemental Table 1 for additional assay details). Additional details can be found in our previous study [8].
This study was approved by the Dartmouth-Hitchcock Institutional Review Board.
3. Results
3.1. Performance characteristics
The Roche, Abbott, and Beckman assays all demonstrated a specificity of 100%, with all 105 pre-SARS-CoV-2 samples (105/105) and samples from SARS-CoV-2 RT-PCR negative inpatients (24/24) producing negative results (Table 1). Sensitivity differed between assays, with the Abbott assay reporting the highest sensitivity of 98% (170/172 inpatients and 19/20 convalescent donors). The Abbott assay was also the most sensitive at all shorter time-point intervals (Table 1). The sensitivity of the Beckman assay was 93% (161/172 inpatients 18/20 convalescent donors), with an additional 3/192 samples (2%) resulted as indeterminate. Of these indeterminate patient results, two seroconverted to positive within 1–2 days. The Roche assay was the least sensitive at 90% (154/172 inpatients, 19/20 convalescent donors), but this was largely due to the inclusion of 15 samples from two immunocompromised patients. After the exclusion of these two patients, the sensitivity of the Roche assay was comparable to the Abbott platform at 98%.
3.2. Signal intensity over time
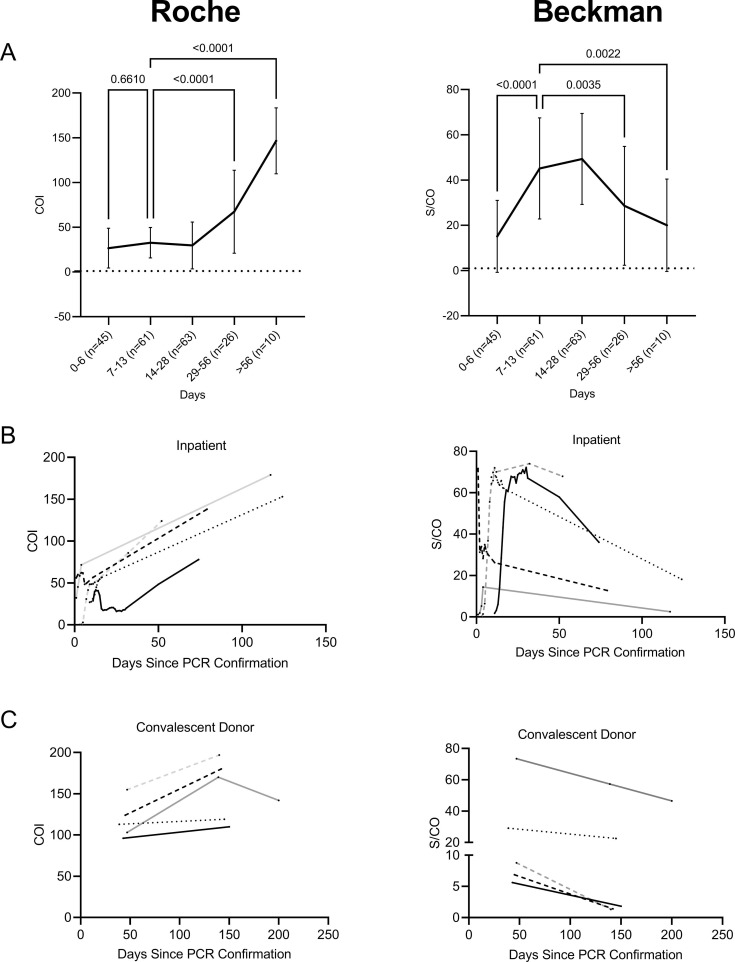
To assess the persistence of nucleocapsid and spike antibodies, samples collected between 0 and 200 days following positive RT-PCR were tested on Roche and Beckman assays (Fig. 1 a). Importantly, most patients did not have serially collected samples present in every time window. The Roche assay signal intensity value increased over time, with the average cutoff index (COI) significantly higher in samples collected between 29 and 56 days (n = 26, P < 0.0001), and > 56 days (n = 10, P < 0.0001) as compared to samples 7–13 days (n = 61) post SARS-CoV-2 positive RT-PCR. The 7–13 day timeframe was chosen as comparator for statistical analysis as most patients are expected to seroconvert within this time (2). In contrast, the signal to calibrator ratio (S/CO) of the Beckman assay peaked at 14–28 days (n = 63) post RT-PCR. The S/CO of samples 29–56 days (n = 26, P = 0.0035) and > 56 days (n = 10, P = 0.0022) were significantly lower than 7–13 days post RT-PCR confirmation.
Fig. 1.
Antibody signal over time in Roche and Beckman serological assays. A: Line graph of average relative signal intensity in Roche (Left, COI) and Beckman (Right, S/CO) platforms over time. The same samples were tested on both platforms, with sample groups divided into 0–6 (n = 45), 7–13 (n = 61), 14–28 (n = 63), 29–56 (n = 26), >56 (n = 10) days post positive PCR. Error bars represent standard deviation. Statistical significance was calculated using a one-way-ANOVA, comparing each group to 8–14 days. B: Signal change in individual inpatients (n = 5) over time in Roche (left) and Beckman (right) assays. C: Signal change in individual convalescent donors (n = 5) over time in Roche (left) and Beckman (right) assays.Each line and color represent a series of plasma or serum samples collected from a single inpatient or convalescent donor.
To further examine signal trends over time, SARS-CoV-2 positive inpatients (Fig. 1b) and convalescent donors (Fig. 1c) with samples collected 51–200 days (median of 139 days) from initial positive RT-PCR result, were tested on both Roche and Beckman assays. The peak COI for the Roche assay of both inpatients (5/5) and convalescent donors (5/5) occurred for samples collected greater than 50 days from initial positive RT-PCR result. The COI intensity continued to climb in all 5/5 inpatients and 4/5 convalescent donors. Only one convalescent donor exhibited a peak COI prior to the final sample collection in this study (Peak COI = 170 at 138 days post positive SARS-CoV-2 RT-PCR, which declined to a COI = 142 at 200 days, Fig. 1c,solid grey line). One inpatient had a decrease of signal intensity after day 14, but the COI intensity subsequently increased (COI = 16 at day 24, COI = 70 at day 73, Fig. 1b, solid black line). The same samples on the Beckman platform exhibited the opposite trend with all inpatients and convalescent donors displaying a peak S/CO prior to 50 days post-positive RT-PCR result, with a subsequent decrease in intensity for samples collected beyond that point.
4. Discussion
In this study, we evaluated the automated Beckman SARS-CoV-2 serological assays and compared it to SARS-CoV-2 assays available from Roche and Abbott. All three assays exhibited 100% specificity but differed regarding sensitivity. Roche had the lowest overall sensitivity, but several samples that were negative via the Roche assay and positive via the Abbott assay came from two immunocompromised patients receiving chemotherapy or anti-CD20 monoclonal therapy. The samples from the patient receiving anti-CD20 monoclonal antibody therapy were positive via the Beckman assay, however the samples from the patient receiving chemotherapy remained consistently negative [8]. Exclusion of the 15 samples from these two patients gave Abbott, Beckman, and Roche overall sensitivities of 98%, 95%, and 98% respectively. Thus, most of the discrepancies between platforms arise from varying initial detection windows, or from immunocompromised patients. It is currently unknown why only the Abbott assay consistently demonstrated reactivity in immunocompromised patients; however, it could be an important consideration for this patient population.
We sought to examine differences in trends between the nucleocapsid and spike antibody response. As the Roche and Abbott assays both detect antibodies to the SARS-CoV-2 nucleocapsid, we elected to examine the persistence of antibody signal in the Roche and Beckman assays. Although the Roche and Beckman assays exhibited similar antibody detection rates, the signal intensities displayed differing trends. The Roche nucleocapsid antibody assay displayed consistently rising signal intensity, with peak average COI occurring greater than 56 days post-positive RT-PCR result. In contrast, the Beckman spike antibody assay displayed peak average S/CO between 15 and 28 days after positive RT-PCR result, with gradually decreasing intensity in subsequent samples. This implies that while nucleocapsid antibodies persist, spike antibodies begin to decline over time. Although it is possible that this is due to analytical platform differences, other studies have observed a similar decline in spike antibody response [9], [10]. While none of the samples became non-reactive or indeterminate on the Beckman platform, samples from three of the convalescent donors had S/CO values of less than 1.8 (S/CO positive threshold is 1.0). Assuming an exponential regression from 15 to 28 days post RT-PCR confirmation, the patients included in our study would not have detectable antibodies within one year. Despite the decreasing signal noted in the Beckman assay, an anamnestic antibody response could confer future protection despite a currently low signal.
This downward trend of spike antibody signal in convalescent donors has been observed in other studies, with significant decreases 90 days post-symptom onset [9], [10]. The observation of decreased spike IgG antibody signal by independent studies using different methods implies that these antibodies do not persist as long as nucleocapsid antibodies. Current vaccines available in the United States at the time of publication elicit an antibody response to the spike protein [11] and will not generate a nucleocapsid antibody response [12], [13]. With this in mind, our results imply that these vaccines may not confer long lasting immunity. However, the loss of antibody signal over time could be an artifact of the assay itself. Our study monitored antibody signal over time in qualitative assays, and a decrease in signal intensity may not correlate with antibody concentration or neutralizing activity. Despite differing trends between assays, all patients and convalescent donors remained positive for spike and nucleocapsid antibodies on their latest collection, which ranged from 51 to 200 days (median = 139 days) post SARS-CoV-2 RT-PCR confirmation.
5. Conclusions
Overall, the Roche, Abbott, and Beckman assays are all highly sensitive and specific for patients who were previously exposed to SARS-CoV-2. The diminishing signal in spike antibodies from the Beckman assay is concerning, but there is currently no consensus on whether spike or nucleocapsid serological tests are an appropriate indicator of lasting or effective immunity against SARS-CoV-2.
Declaration of Competing Interest
Any previous presentation or web description of the manuscript: NA.
List of any “Human Genes“: NA.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2021.05.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Patel A., Jernigan D.B. nCo VCDCRT. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak – United States, December 31, 2019-february 4, 2020. MMWR Morb. Mortal Wkly. Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (sars-cov-2) Clin. Transl. Immunol. 2020;9 doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paltiel A.D., Zheng A., Schwartz J.L. Speed versus efficacy: quantifying potential tradeoffs in covid-19 vaccine deployment. Ann. Intern. Med. 2021 doi: 10.7326/M20-7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi N., Cortade D.L., Ng E., Wang S.X. Diagnostics for sars-cov-2 detection: a comprehensive review of the fda-eua covid-19 testing landscape. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta N.K., Mazumdar K., Gordy J.T. The nucleocapsid protein of sars-cov-2: a target for vaccine development. J. Virol. 2020;94 doi: 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong Y., Ulasli M., Schepers H., Mauthe M., V'Kovski P., Kriegenburg F. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J. Virol. 2020;94 doi: 10.1128/JVI.01925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222:206–213. doi: 10.1093/infdis/jiaa273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubbard J.A., Geno K.A., Khan J., Szczepiorkowski Z.M., de Gijsel D., Ovalle A.A. Comparison of two automated immunoassays for the detection of sars-cov-2 nucleocapsid antibodies. J. Appl. Lab. Med. 2020 doi: 10.1093/jalm/jfaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perreault J., Tremblay T., Fournier M.J., Drouin M., Beaudoin-Bussieres G., Prevost J. Waning of sars-cov-2 rbd antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136:2588–2591. doi: 10.1182/blood.2020008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang O.O., Ibarrondo F.J. Loss of anti-sars-cov-2 antibodies in mild covid-19. Reply. N. Engl. J. Med. 2020;383:1697–1698. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 11.Dai L., Gao G.F. Viral targets for vaccines against covid-19. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (sars-cov-2) in individuals with and without covid-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin. Chim. Acta. 2021;518:9–16. doi: 10.1016/j.cca.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorschug A., Frickmann H., Schwanbeck J., Yilmaz E., Mese K., Hahn A. Comparative assessment of sera from individuals after s-gene rna-based sars-cov-2 vaccination with spike-protein-based and nucleocapsid-based serological assays. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.