Figure 1.
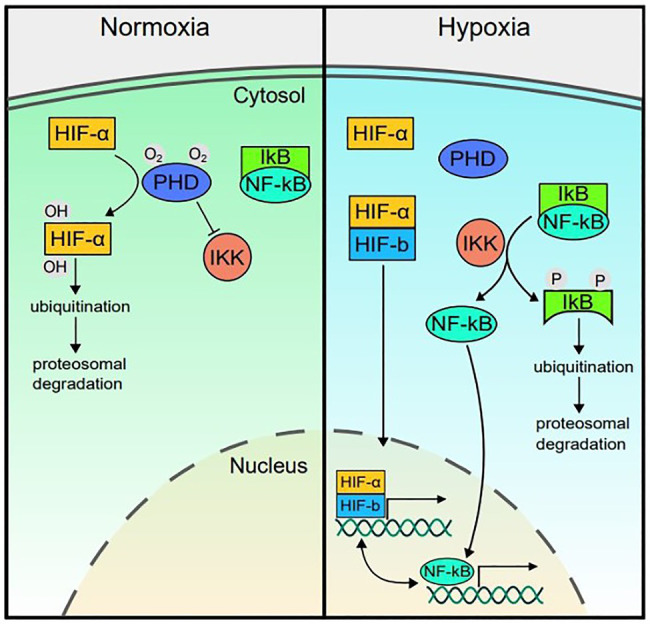
Hypoxia inducible factor (HIF)-nuclear factor (NF)-kappa B (NF-κB) crosstalk. In normoxic conditions, prolyl hydroxylases (PHDs) hydroxylate HIF-α and the IKKβ subunit of the IκB kinase (IKK) complex, marking them for degradation and thereby reducing transcriptional activity of HIF and repressing (but not completely blocking) NF-kB activity. In hypoxia, PHD activity decreases since it utilizes oxygen as a cofactor. Therefore, HIF-α is stabilized and can dimerize with the constitutively active HIF-β subunit. The complex translocates to the nucleus to upregulate expression of genes involved in the hypoxia response. In hypoxia, with reduced PHD activity, the rate of IKK degradation of IκB increases, releasing repression of NF-κB and allowing it to translocate to the nucleus at higher rates and upregulate inflammatory gene expression. PHD, prolyl hydroxylase; HIF, hypoxia-inducible factor; NF-κB, Nuclear factor kappa B; IKK, IκB kinase complex (composed of IKK-α, IKK-β, and IKK-γ subunits); and IκB, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor.
