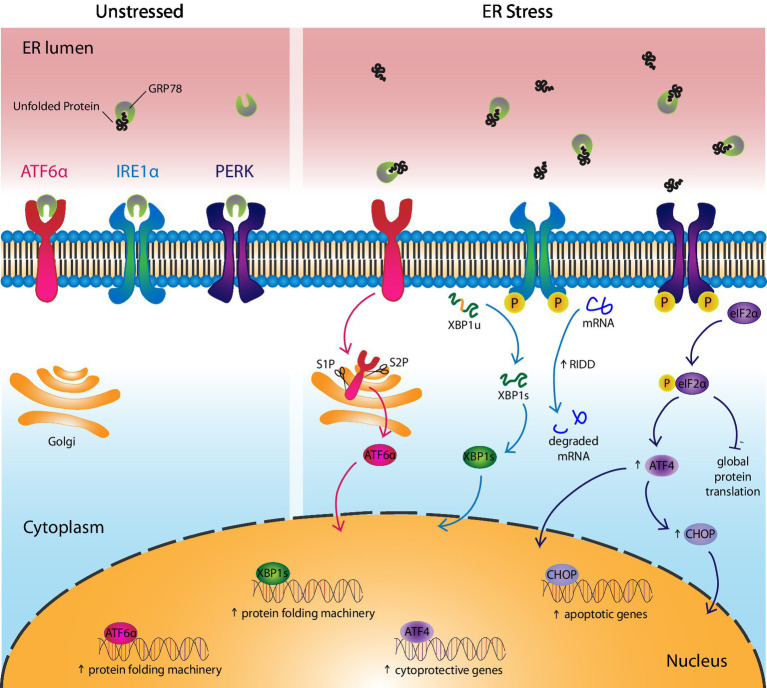
Figure 1.
Cells respond to ER stress via UPR activation. The UPR consists of three canonical receptors, activating transcription factor 6α (ATF6α), inositol-requiring enzyme 1α (IRE1α), and protein kinase R-like ER kinase (PERK). (Left) Under unstressed conditions, GRP78, the UPR ligand and chaperone, binds the UPR receptors to maintain them in their inactive state. (Right) In response to ER stress, GRP78 leaves the UPR receptors and assists in the folding of unfolded proteins, thereby activating the receptors. More specifically, ATF6α translocates to the Golgi where it is cleaved by enzymes, site-1 protease and site-2 protease, before entering the nucleus as a transcription factor that upregulates genes involved in protein folding. IRE1α undergoes autophosphorylation and activates its endoribonuclease, which splices unspliced XBP1(u) mRNA into spliced XBP1(s). XBP1s protein acts as a transcription factor that upregulates genes involved in protein folding. IRE1α also reduces ER stress by splicing mRNAs into non-functional transcripts before they can be translated, a process called regulated IRE1-dependent decay. PERK autophosphorylates then phosphorylates eIF2α, which inhibits protein translation, with the exception of ATF4-regulated genes like CHOP. ATF4 upregulates cytoprotective genes and in the case of chronic ER stress, it induces apoptosis via CHOP.