Figure 3.
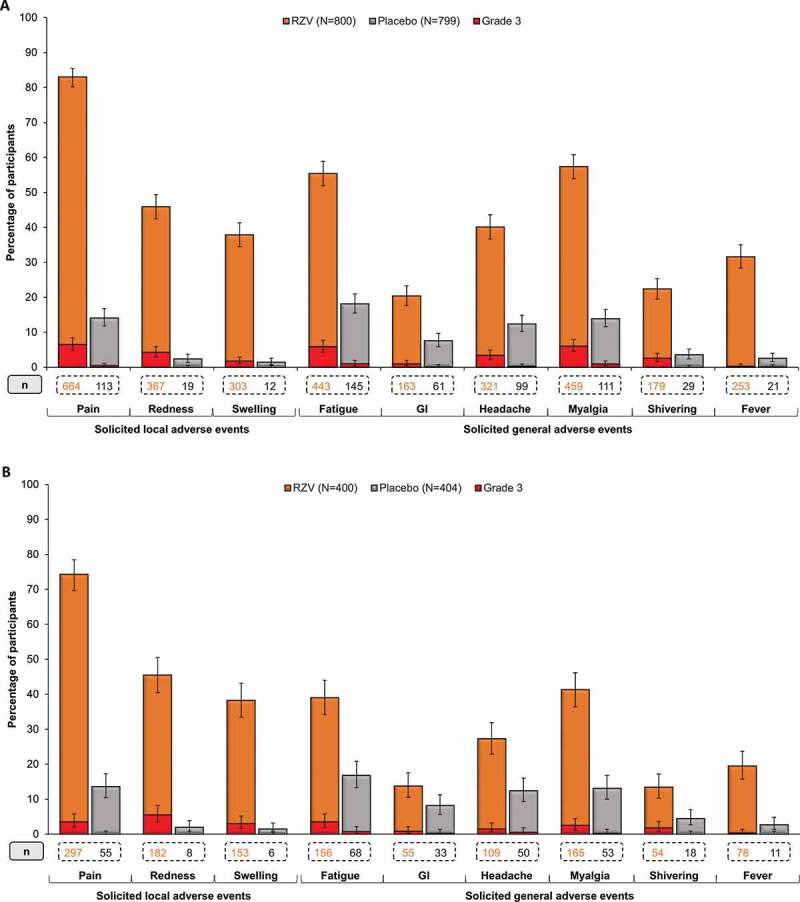
Solicited local and general adverse events (AEs) reported within 7 days after vaccination in (A) ZOE-50 Asian population ≥50 YOA and (B) pooled ZOE-50/70 Asian population ≥70 YOA (TVC diary card sub-cohort)
RZV, participants receiving the adjuvanted recombinant zoster vaccine; Placebo, participants receiving placebo; TVC, total vaccinated cohort; YOA, years of age; GI, gastrointestinal symptoms; N, total number of participants; n, number of participants reporting the event. Error bars depict 95% confidence intervals.
