Figure 4.
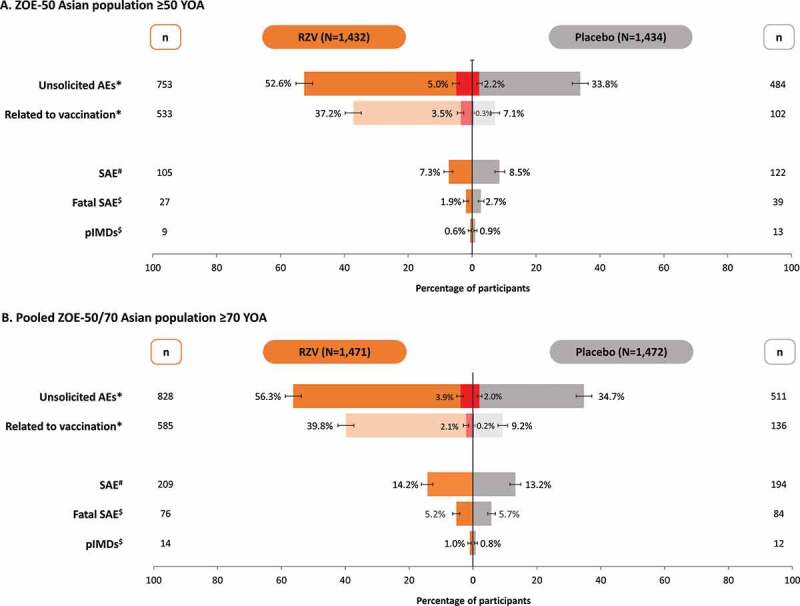
Unsolicited adverse events, serious adverse events, and potential immune-mediated diseases in (A) ZOE-50 Asian population ≥50 YOA and (B) pooled ZOE-50/70 Asian population ≥70 YOA (total vaccinated cohort)
RZV, participants receiving the adjuvanted recombinant zoster vaccine; Placebo, participants receiving placebo; YOA, years of age; AE, adverse events; SAE, serious adverse events; pIMD, potential immune-mediated disease; N, total number of participants; n, number of participants reporting the event. *events recorded during the 30 days post-vaccination period. #Events recorded up to 12 months post-second dose. $Events recorded during the entire study period. See text for additional details.
Note: Error bars represent 95% confidence interval. Red bars indicate the number of participants with grade 3 unsolicited AEs.
