Figure 2.
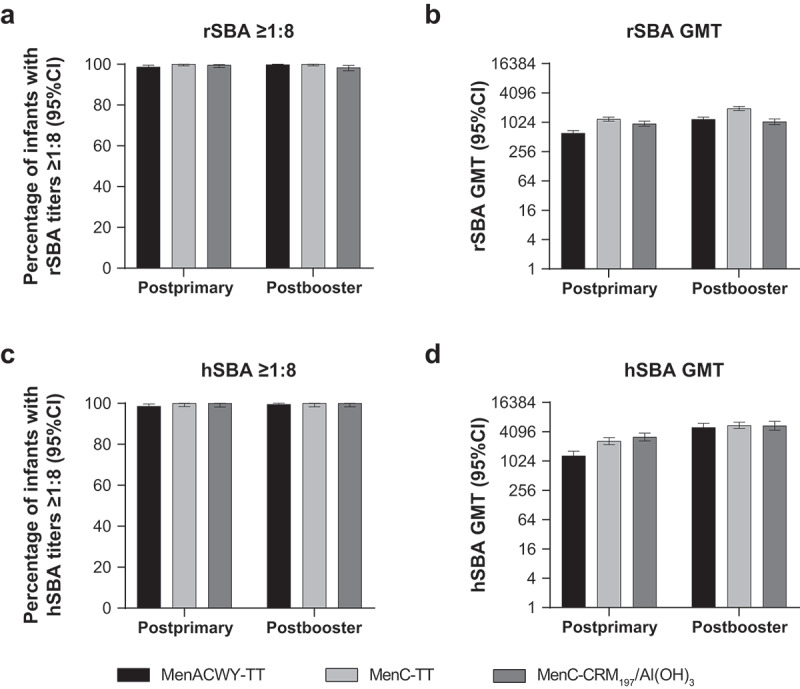
Serum bactericidal antibody measurements against serogroup C at 1 month postprimary and postbooster vaccination in infants vaccinated with MenACWY-TT, MenC-TT, or MenC-CRM197/Al(OH)3 at 2, 4, and 12 months of age.60 (a) Percentage of infants with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of infants with hSBA titers ≥1:8, and (d) hSBA GMTs.
Subjects had blood samples collected at 1 month (range, 21–48 days) after the second primary dose (primary ATP cohort) and the booster dose (booster ATP cohort). Panel A. Postprimary, n = 455–457; postbooster, n = 446–463. Panel B. Postprimary, n = 455–457; postbooster, n = 446–463. Panel C. Postprimary, n = 202–226; postbooster, n = 216–221. Panel D. Postprimary, n = 202–226; postbooster, n = 216–221. ATP = according to protocol; GMT = geometric mean titer; hSBA = serum bactericidal antibody using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y vaccine conjugated to tetanus toxoid as a carrier protein; MenC-CRM197/Al(OH)3 = meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum hydroxide; MenC-TT = meningococcal serogroup C vaccine conjugated to tetanus toxoid; rSBA = serum bactericidal antibody using baby rabbit complement.
