Figure 5. Comparison of the P. aeruginosa, N. gonorrhoeae and N. meningitidis Type IV pilus structures.
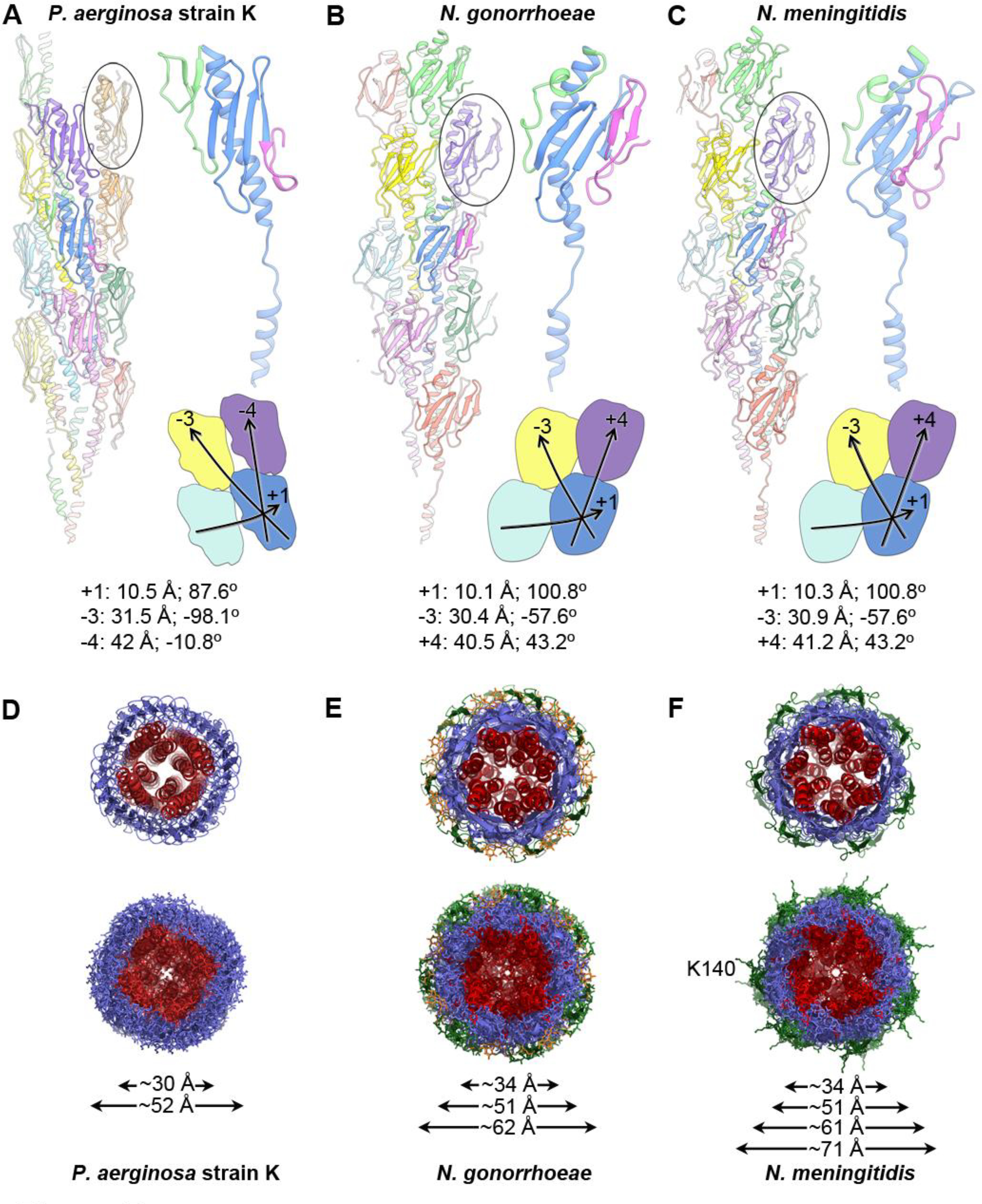
(A) A ribbon representation of the P. aeruginosa strain K T4P is shown on the left with the globular domain of a pilin subunit shown in side view circled. The central blue subunit is enlarged on the right, with its αβ-loop colored green and its D-region colored magenta. The general shape and packing of the globular domains is shown below, as are the symmetry parameters for the reconstruction. (B, C) The N. gonorrhoeae and N. meningitidis T4P structures, packing and symmetry are shown as in (A). The shape and packing of the globular domains is substantially different for PaK T4P than for the Neisseria pili. (D-F) End views of the PaK (D), N. gonorrhoeae (E) and previously determined N. meningitidis (F) T4P structures show similar diameters for the α-helix/β-sheet components for all three pili, with the β-hairpins and post-translational modifications accounting for the larger diameters of the Neisseria pili. The α1s are colored red, the hypervariable β-hairpins present on the Neisseria pili are shown in green, and the post-translational modifications at Ser63 and Ser68 of N. gonorrhoeae are colored orange. Lys140 protrudes from the β-hairpins in N. meningitidis T4P. Diameter measurements are the maximum Cα-Cα distances, with the exception of the largest diameter reported for Nm T4P, which includes the protruding Lys140 side chains.
