Abstract
In this secondary analysis of data from a double‐blind randomized controlled trial (clinicaltrials.gov identifier: NCT00133744) of micronutrient supplementation (multiple micronutrients [MMN], iron–folic acid [IFA] and folic acid [FA] alone), we examined the potential modifying effect of gestational age at enrolment on the association of antenatal supplementation and pregnancy‐induced hypertension (PIH). We included 18,775 nulliparous pregnant women with mild or no anaemia who were enrolled at 20 weeks of gestation or earlier from five counties of northern China. Women were randomly assigned to receive daily FA, IFA or MMN from enrolment until delivery. We used logistic regression to evaluate the association between PIH and timing of micronutrient supplementation. The incidence of PIH was statistically significantly lower among women who began MMN supplementation before 12 gestational weeks compared with women who began MMN supplementation at 12 weeks or later (RR = 0.74, 95% CI: 0.60–0.91). A similar protective effect was observed for both early‐onset (<28 weeks, RR 0.45, 0.21–0.96) and late‐onset of PIH (≥28 weeks, RR 0.77, 0.63–0.96). No statistically significant association was observed between PIH occurrence and timing of supplementation for FA or IFA. Maternal MMN supplementation and antenatal enrolment during the first trimester of pregnancy appeared to be of importance in preventing both early‐ and late‐onset of PIH.
Keywords: antenatal enrolment, double‐blind RCT, large cohort study, micronutrients, pregnancy‐induced hypertension, prenatal nutrition, United Nations multiple micronutrient antenatal preparation
Key messages.
This research is a secondary analysis of a large RCT, investigating the relationship between micronutrients supplementation during pregnancy and pregnancy‐induced hypertension (PIH) among Chinese women.
Our results supported that maternal multiple micronutrient (MMN) supplementation during the first trimester appeared to be of particular importance for the prevention of PIH.
Our findings may provide robust evidence for nutritional supplementation during pregnancy and contribute data important with respect to the modification of public health policies for the prevention of PIH.
1. INTRODUCTION
Micronutrients, including vitamins and minerals, are needed by the body in very small quantities but can impact immediate, short‐term and long‐term human health status. Many women in low‐ and middle‐income countries are malnourished, especially in pregnancy due to the increased demands of micronutrients by the fetus and placenta. Compared with single nutrient supplementation, multiple micronutrients (MMNs) have been suggested as an effective way to achieve multiple benefits for pregnant women and infants (Allen, 2005). The UNICEF/WHO/UNU has designed a MMN supplement (UNIMMAP, United Nations Multiple Micronutrient Antenatal Program) that contain 15 vitamins and minerals with recommended dietary allowance for pregnant and lactating women in 1999 (Unicef, 1999). In order to evaluate the effect of MMN supplementation on pregnancy complications, a variety of studies have been conducted (Fall et al., 2009; Haider et al., 2011; Rumiris et al., 2006). Many studies have shown that MMN supplementation during pregnancy has significant benefit in reducing small for gestational age and preterm births (Haider et al., 2011; Li et al., 2017). However, there is little evidence linking MMN supplementation to the prevention of pregnancy‐induced blood pressure disorders.
Pregnancy‐induced hypertension (PIH), defined as hypertension that occurs after 20 weeks of pregnancy, may be accompanied by proteinuria or seizures in serious cases (Ananth & Basso, 2010). PIH is one of the most serious complications of pregnancy including gestational hypertension and preeclampsia, which effects 5%–7% of pregnancies worldwide (Lindheimer et al., 2008). PIH has a major impact on both the pregnant woman and her fetus. PIH is strongly associated with caesarean section, renal dysfunction, placental abruption and cardiovascular morbidity (Bellamy et al., 2007; Wang et al., 2013); the fetus also has a higher risk of adverse pregnancy outcomes such as intrauterine growth restriction and low birth weight (Sibai, 2006). Although there have been many studies conducted to explain and predict the occurrence of PIH, the understanding of the aetiology is still poor (Steegers et al., 2010). Previous studies have shown that oxidative stress and endothelial cell damage are related to the occurrence of preeclampsia (Henriques et al., 2014). Furthermore, low levels of serum antioxidants have been associated with an increase in oxidative stress and endothelial cell damage. Therefore, supplementation with antioxidant nutrients, such as copper (Lindheimer et al., 2008), zinc (Jain et al., 2010), magnesium or calcium (Elmugabil et al., 2016; Ephraim et al., 2014; He et al., 2016), as well as Vitamins C and E (Chappell et al., 1999; Lorzadeh et al., 2020), is considered to potentially protect against preeclampsia in pregnant women. In regard to iron, the relationship between serum iron level and oxidative stress is inconsistent, some studies have found that pregnant women with gestational hypertension have lower serum iron levels than those with normotension (Ahsan et al., 2013; Sarwar et al., 2013), whereas other studies have not found this association (Fenzl et al., 2013; Siddiqui et al., 2011).
A double‐blinded, individual randomized controlled trial (RCT) (clinicaltrials.gov identifier: NCT00133744) was conducted in rural areas of northern China during 2006–2009 to evaluate whether prenatal supplementation with MMN or iron plus folic acid (IFA) versus folic acid (FA) could reduce perinatal mortality (primary outcomes) and other health outcomes for pregnant women and fetuses (Liu et al., 2013). In a previous secondary analysis of the trial data, we examined the association of prenatal supplementation with PIH and did not find a significant difference in overall PIH across the three supplementation groups (Chen et al., 2018). However, the timing of prenatal supplementation may be important in understanding the preventive effects. A healthy diet starting from early pregnancy, or before, is important for the prevention of neural tube defects and child growth (Cetin et al., 2010; Khan et al., 2011). To our knowledge, the importance of timing of prenatal supplementation on risk of PIH has not been well investigated. In this post hoc secondary analysis, we aim to exam whether the incidence of PIH and its subtypes vary by timing of supplementation (<12 gestational weeks vs. ≥12 gestational weeks) for each of the three supplement groups.
2. METHODS
2.1. Study design and location
The initial study was designed to be a double‐blinded, RCT, with three study groups (MMN, IFA, FA) in five counties in northern China's Hebei Province. All the project counties are within 2–3 h of drive to Beijing. The local population was homogeneous with little mobility. Health facilities and services were similar across these counties. Basic health services were provided through the health care network of three levels (county, township and village). Full details of the original trial have been published elsewhere (Liu et al., 2013).
2.2. Recruitment and menstrual monitoring
Before and during the recruitment period, the project was widely publicized through pamphlets, posters and television channels. Township doctors also informed fertile women of this study at regular consultations. If women were interested in the study, they were given a calendar to record their menstrual periods. Township or village health workers visited or called these women at least once a month to record the dates of their menstruation. When these women thought they were pregnant or had missed menstrual period more than 1 week, they were advised to do a pregnancy test in village clinics or hospitals. Once the pregnancy was confirmed, women were invited to enter this RCT before gestation of 20 weeks.
2.3. Enrolment and intervention
The eligibility and inclusion of subjects were ultimately determined by the hospital's prenatal diagnosis. Women who met the criteria below were eligible to enter the trial: (1) resided and carried out prenatal examination in one of the five counties, (2) greater than or equal to 20 years old, (3) two or more months' menstrual dates recorded before they were pregnant, (4) were nulliparous, (5) were not more than 20 gestational weeks confirmed via last menstrual period, (6) were legally competent, (7) haemoglobin level was above 10.0 g/dl, (8) no other micronutrient supplements were consumed within the last six months except FA, (9) consented to participate. From May 2006 to April 2009, eligible pregnant women were enrolled and individually randomized in a 1:1:1 ratio into three groups to receive different daily supplements as follows: FA (0.4 mg); IFA, FA (0.4 mg) plus iron (30 mg); and MMN (UNIMMAP supplement has the same amount of iron and FA as the IFA). The composition of micronutrients and amounts provided in the UNIMMAP was presented in the Supplementary Table S1. Regardless of lot number or content, all pills had the same size, shape and colour. Detailed information about randomization and masking was described in our primary manuscript (Liu et al., 2013).
2.4. Participation and data collection
Women were followed up from the enrolment before 20 gestational weeks until 4–8 weeks of post‐partum visit. Pregnant women were asked to attend at least one prenatal care in the first 12 weeks of pregnancy, once every 4 weeks from 12 to 28 weeks, once every 2 weeks from 28 to 36 weeks and once a week after 36 weeks. Each month they were asked to send their previous month pill bottles to the clinic. Health care workers counted the number of pills remaining, calculate how many pills were consumed and then distribute pills for the next month. Physicians measured relevant indicators and collected data according to the field operation manual. Maternal haemoglobin, birth weight of the baby, maternal and infant weight were measured using standard equipment and methods (Liu et al., 2013). All the collected data were recorded by local health workers and entered into the electronic reproductive health monitoring system at the time of the visit.
2.5. Definition of PIH
The blood pressure of pregnant woman was measured with a mercury sphygmomanometer by trained physicians during each prenatal care. Appropriate‐sized cuff bladder size was determined according to the pregnant woman's arm circumference in each measurement. Blood pressure was measured in the right arm with a standard mercury sphygmomanometer device, and its value was observed on 2 or more consecutive occasions with an interval of ≥2 min. PIH is defined as any occurrence of either systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg starting at or after 20 weeks of gestation among women with previously normal blood pressure prior to 20 weeks (Ananth & Basso, 2010; Jennifer Ribowsky, 2012). PIH was classified into two categories: early‐onset (onset at 20 to <28 weeks of gestation) and late‐onset (onset at ≥28 weeks of gestation) (Li et al., 2013).
2.6. Statistical analysis
Any woman with one high BP measurement prior to 20 weeks was excluded from all analyses. To be eligible for the subanalysis of early‐onset PIH, each woman had to have blood pressure measured before 20 weeks of gestation and between 20 and 28 weeks of gestation. For the analysis of late‐onset PIH, each woman had to have blood pressure measured during the first two time periods and after 28 weeks of gestation. Women who were found to have early‐onset hypertension were also excluded from analysis of late‐onset hypertension.
In order to evaluate the effectiveness of the randomization, we compared the baseline characteristics of participants across three treatment groups, including women's age, height, body mass index (BMI) (weight [kg]/height [m]2), ethnicity, education, occupation and gestational week at registration. Compliance was calculated by dividing the number of supplements actually consumed by the number of supplements that should have been consumed (i.e., the number of days between enrolment and delivery). The effects of the intervention were analysed based on an intent‐to‐treat principle. All of the participants with known outcomes were included in our analysis regardless of compliance. We calculated the incidence of PIH and subtypes (early‐ vs. late‐onset PIH) for the three different supplement groups respectively by gestational age of enrolment. To examine possible effect modification of PIH and enrolment timing, we added an interaction term between supplement groups and enrolment category. Interactions with a two‐sided P < 0.10 were considered to be significant. We further conducted stratified analysis because effect modification was significant. Because the basic characteristics of women were well balanced across different supplement groups, univariate logistic regression was used to estimate the risk ratios by supplement group for PIH. All data were analysed using the SPSS software (ver. 20; SPSS Inc., Chicago, IL, USA).
2.7. Ethical considerations
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the institutional review boards of the Centers for Disease Control and Prevention, Atlanta, Georgia, and Peking University, Beijing, China. Verbal informed consent was obtained from all subjects. Verbal consent was witnessed and formally recorded.
3. RESULTS
Figure 1 details the enrolment, randomization and follow‐up of the participants and the participant selection for the current analysis. Among the 18,963 pregnant women considered for enrolment, 120 refused to participate in this study, and 68 were not eligible. After these exclusions, a total of 18,775 women were randomized into three treatment groups, 6,261 took only FA supplements (FA group), 6,252 took IFA supplements (IFA group) and 6,262 took MMN supplements (MMN group).
FIGURE 1.
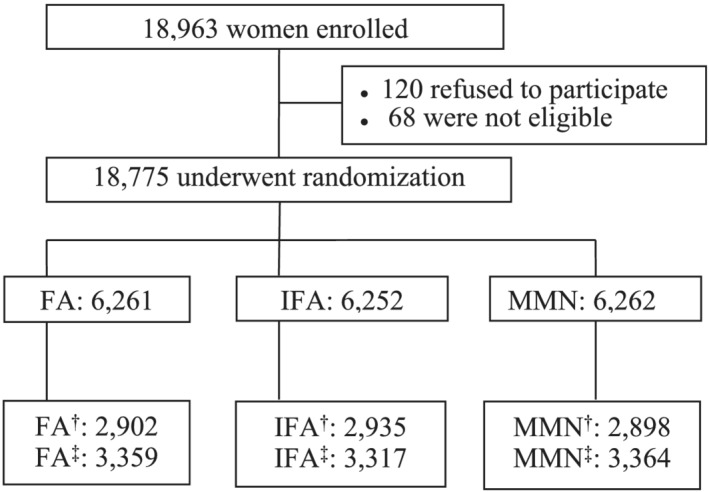
Flowchart of participants
Table 1 shows baseline characteristics of study population across in the six different groups (type of supplement and gestation age at enrolment). Overall, most participants were young women with average age about 23 years old with a junior high school education or lower. Almost 99% of the participants were of Han ethnicity, and about 90% were farmers. For participants enrolled from 12 to 20 weeks of pregnancy, the average gestational age at enrolment was 16 weeks in all three groups; for those enrolled before 12 weeks, the average gestational age was 8 weeks in all three groups. The average BMI of women who enrolled in the study before 12 weeks of gestation was slightly lower than those who enrolled during the 12th week or later because of the weight gain during pregnancy. There was significant difference in the occurrence of fetal loss and preterm birth as risk at different groups. Although median compliance was the same for each group (97%), the average number of supplements consumed was lower in those enrolled at ≥12 weeks (median 151–154 supplements consumed) compared with those enrolled earlier (208–209 supplements consumed).
TABLE 1.
Baseline maternal characteristics and compliance by enrolment timing and supplements categories in northern China, 2006–2009
| Characteristics | Enrolment at ≥12 gestational weeks | Enrolment at <12 gestational weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA (N = 2,902) | IFA (N = 2,935) | MMN (N = 2,898) | FA (N = 3,359) | IFA (N = 3,317) | MMN (N = 3,364) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Gestational week at enrolment a | 16.1 (2.6) | 16.2 (2.5) | 16.2 (2.5) | 8.3 (2.1) | 8.3 (2.1) | 8.3 (2.1) | ||||||
| Fetal loss <28 gestational weeks | 33 | 1.2 | 30 | 1.0 | 49 | 1.7 | 211 | 6.4 | 203 | 6.2 | 206 | 6.2 |
| Preterm birth | 209 | 7.4 | 214 | 7.4 | 190 | 6.7 | 144 | 4.7 | 126 | 4.1 | 118 | 3.8 |
| Compliance b , c | 97.0 | 97.0 | 97.0 | 97.0 | 97.0 | 97.0 | ||||||
| Supplements consumed c | 154 | 153 | 151 | 209 | 208 | 208 | ||||||
| Age at pregnancy (year) a | 23.5 (3.0) | 23.4 (2.9) | 23.3 (2.8) | 23.5 (2.9) | 23.5 (2.8) | 23.5 (2.8) | ||||||
| Height (cm) a | 160.1 (4.4) | 160.1 (4.5) | 160.1 (4.5) | 160.0 (4.6) | 160.3 (4.6) | 160.2 (4.5) | ||||||
| BMI at enrolment a , d | 22.6 (2.9) | 22.4 (2.8) | 22.5 (2.8) | 22.1 (2.8) | 22.1 (2.9) | 22.2 (3.0) | ||||||
| Education | ||||||||||||
| Primary school or lower | 546 | 18.8 | 510 | 17.4 | 501 | 17.3 | 610 | 18.2 | 615 | 18.5 | 642 | 19.1 |
| Junior high | 2,321 | 80.0 | 2,374 | 80.9 | 2,348 | 81.0 | 2,690 | 80.1 | 2,643 | 79.7 | 2,677 | 79.6 |
| High school or higher | 35 | 1.2 | 51 | 1.7 | 49 | 1.7 | 59 | 1.8 | 59 | 1.8 | 45 | 1.3 |
| Ethnicity | ||||||||||||
| Han | 2,872 | 99.0 | 2,903 | 98.9 | 2,870 | 99.0 | 3,308 | 98.5 | 3,281 | 98.9 | 3,322 | 98.8 |
| Others | 30 | 1.0 | 32 | 1.1 | 28 | 1.0 | 51 | 1.5 | 36 | 1.1 | 42 | 1.2 |
| Occupation | ||||||||||||
| Farmer | 2,635 | 90.8 | 2,678 | 91.2 | 2,636 | 91.0 | 3,039 | 90.5 | 3,013 | 90.8 | 3,046 | 90.5 |
| Others | 267 | 9.2 | 257 | 8.8 | 262 | 9.0 | 320 | 9.5 | 304 | 9.2 | 318 | 9.5 |
Abbreviations: BMI, body mass index; FA, folic acid; IFA, iron–folic acid; MMN, multiple micronutrients.
Values are expressed as mean (standard deviation).
Compliance was calculated as the number of supplements consumed divided by days supplements was expected to be consumed.
Values are expressed as medians.
Weight (kg)/height (m)2.
Table 2 shows the incidence of PIH according to micronutrient supplements and timing of enrolment. PIH incidence for women who started consuming FA, IFA and MMN during the 12th week of gestation or later was 7.4%, 5.8% and 6.8%, and before the 12th gestational week were 6.2%, 5.9% and 5.1%, respectively. There was a significant modification effect of supplement group by gestational age at enrolment (P interaction = 0.01) on PIH incidence. Women who started consuming FA or IFA before the 12th gestational week did not show significantly reduced risks of PIH and its two categories relative to taking those two supplementations from the 12th week, respectively. While compared with MMN group enrolled ≥12 gestational weeks, early consumption of MMN before the 12th gestational week showed a 26% (RR = 0.74, 95% CI: 0.60–0.91) risk reduction for PIH. For subtypes of late‐ and early‐onset PIH, the early consumption of MMN before 12th gestational week also showed similar significantly protective effects by reducing risks 23% (RR = 0.77, 95% CI: 0.62–0.96) and 55% (RR = 0.45, 95% CI: 0.21–0.96), respectively.
TABLE 2.
Incidence of PIH according to timing of enrolment and supplements categories in northern China, 2006–2009
| PIH category | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Late‐onset (≥28 weeks) | Early‐onset (<28 weeks) | ||||||||||
| No. of cases | Incidence, % | RR | 95% CI | No. of cases | Incidence ‡, % | RR | 95% CI | No. of cases | Incidence b , % | RR | 95% CI | |
| Enrolment at ≥12 gestational weeks a | ||||||||||||
| FA | 216 | 7.4 | Ref | 198 | 6.9 | Ref | 18 | 0.7 | Ref | |||
| IFA | 170 | 5.8 | Ref | 164 | 5.6 | Ref | 6 | 0.2 | Ref | |||
| MMN | 197 | 6.8 | Ref | 178 | 6.2 | Ref | 19 | 0.7 | Ref | |||
| Enrolment at <12 gestational weeks | ||||||||||||
| FA | 209 | 6.2 | 0.83 | 0.68, 1.01 | 196 | 5.9 | 0.84 | 0.69, 1.04 | 13 | 0.4 | 0.62 | 0.30, 1.26 |
| IFA | 196 | 5.9 | 1.02 | 0.83, 1.26 | 182 | 5.5 | 0.98 | 0.79, 1.22 | 14 | 0.4 | 2.07 | 0.79, 5.39 |
| MMN | 172 | 5.1 | 0.74 | 0.60, 0.91 | 162 | 4.8 | 0.77 | 0.62, 0.96 | 10 | 0.3 | 0.45 | 0.21, 0.96 |
Abbreviations: CI, confidence interval; FA, folic acid; IFA, iron–folic acid; MMN, multiple micronutrients; RR, relative risk.
Reference group for RR estimation.
The calculation for incidence did not include other subtypes of PIH in denominator.
4. DISCUSSION
In this large double‐blind RCT of prenatal nutritional supplementation conducted in a well‐nourished population in rural northern China, we examined the association between PIH and timing of micronutrient supplementation (MMNs, IFA and FA alone) from a secondary analysis. Compared with later supplementation, consuming MMN during the first trimester of pregnancy was associated with 23%–55% reduction in the incidence of PIH and its two subtypes, early‐ and late‐onset PIH. However, there was no association between PIH and timing of either IFA or FA supplementation.
With the increasing use of prenatal MMN and IFA supplements, more attention has being paid to the effects of these supplements on pregnancy complications compared with FA supplementation only. While there have been few RCTs studies which evaluate the effects of MMN or IFA supplementation on overall PIH or preeclampsia. A small randomized clinical trial conducted among 30 pregnant women with low antioxidant status found that MMN (non‐UNIMMAP formulation) reduced the risk of PIH compared with IFA among pregnant women with low antioxidant status. (Rumiris et al., 2006). However, another two randomized clinical trials in China and Mexico suggested that MMN (UNIMMAP formulation) did not show a protective effect for the prevention of preeclampsia compared with a placebo group among well‐nourished women (Chen et al., 2018). Pooled analysis of RCTs also found that supplementation with Vitamins C and E were not associated with a reduced risk of preeclampsia or gestational hypertension (Conde‐Agudelo et al., 2011; Rossi & Mullin, 2011). The inconsistent conclusions may be due to differences in the timing of supplementation among those studies.
Although many researchers have tried to investigate the relationship between MMN or IFA use during pregnancy with PIH, few studies have examined the effect of timing of supplementation on PIH, especially according to PIH subtype. The timing of enrolment for prenatal care and start of supplementation is a crucial time window that may influence placenta development; this may in return affect maternal health status (Chen et al., 2018). A healthy diet before and in the early pregnancy period is important in reducing pregnancy complications and adverse outcomes, ranging from infertility to fetal defects and long‐term diseases (Cetin et al., 2010; Khan et al., 2011). In our study, the incidences of fetal loss and preterm birth were unbalanced among different group interventions. Occurrence of fetal loss decreased at later enrolment for prenatal care and the start of supplementation (Cetin et al., 2010). However, duration of pregnancy has been hypothesized to be the ultimate consequence of preterm birth in the very earliest stages of pregnancy. Our colleagues once found that early prenatal enrolment and micronutrient use during first trimester of pregnancy appeared to be of particular importance for prevention of preterm birth from the same trial data as our study (Li et al., 2017).
MMN contains many kinds of antioxidants, such as Vitamins C D, and E, copper and selenium. Therefore, compared with FA supplements alone, MMN supplementation during early pregnancy may theoretically help prevent PIH by providing additional antioxidants. In a prospective pilot study, low serum zinc levels measured in the first‐trimester were associated with a reduced risk of gestational hypertension among nulliparous women (adjusted odds ratio = 0.93, 95% CI: 0.87–0.99) (Tande et al., 2013). Both elevated serum iron levels (Toblli et al., 2012; Viteri et al., 2012) or iron deficiency (Casanueva & Viteri, 2003; Henriques et al., 2014; Ramakrishnan et al., 2013) have been found in connection with oxidative stress endothelial cell function, but there were also some studies that did not find relationship between serum iron level and oxidative stress (Ahsan et al., 2013; Fenzl et al., 2013; Sarwar et al., 2013; Siddiqui et al., 2011). We speculate that single or some specific nutritional components of MMN, especially copper and zinc, might help to prevent PIH by reducing oxidative stress or endothelial cell damage (Conde‐Agudelo et al., 2011; Henriques et al., 2014). The combined effects of MMN components may play an important role in the homeostasis of oxidative–antioxidative response. However, this is only conjecture and further studies are needed.
As women who enrolled early in the trial also consumed more MMN, we could not separate out whether the protective effect of early enrolment was due to earlier introduction of MMN or to a higher dose of MMN. Furthermore, although the demographic characteristics of early and late enrolees were similar, we could not adjust for residual confounding from unmeasured health‐seeking characteristics leading to earlier pre‐natal care or other effects of earlier prenatal care. Another limitation is that we could not distinguish between pregnancy hypertension and preeclampsia because we did not measure urinary protein or renal function. In addition, even though the average number of blood pressure measurements was more than seven for all the women, some abnormal blood pressure measurements may have been missed. Finally, our survey was conducted in a population of normal weight women with no or mild anaemia who had good access to medical care (almost all deliveries were in the hospital), and the results were not generalizable to malnourished populations, especially for populations with high levels of overweight and obesity, or those with high levels of anaemia.
Our study had several strengths. The study was a double‐blind, large RCT and individual based; the baseline characteristics across groups were well‐balanced. To our knowledge, it is the first large RCT of micronutrient supplementation to examine the incidence of PIH by timing of enrolment. Supplement consumption was confirmed by health workers monthly and the average compliance reached 97%; the loss to follow‐up was less than 1%. The combination of self‐monitoring of menstruation, monthly visits by village health workers and timely laboratory confirmation of pregnancy allowed early detection of pregnancy and accurate record of gestational age. Finally, unlike many trials conducted in poor populations in developing countries, our trial was generalizable to a well‐nourished population of nonanemic or mildly anaemic women with good access to health care.
In conclusion, our study indicated that, comparing with later supplementation, daily supplementation with MMN during first trimester of pregnancy could protect against PIH. However, there was no association between timing of supplementation with either IFA or FA and protection against PIH.
CONFLICT OF INTEREST
All authors read and approved the final manuscript and none of conflict of interest or competing interest consists.
CONTRIBUTIONS
YL and NL contributed equally to this manuscript. YL and NL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YL, NL, ZM and RY. Acquisition of data: YL, NL, ZM and JL. Analysis and interpretation of data: YL, NL, ZM, ZL, JL, HL, YZ and MS. Drafting of the manuscript: YL, NL, and ZM. Critical revision of the manuscript for important intellectual content: YL, NL, ZM, RY, ZL and MS. Statistical analysis: YL, NL and ZM. Administrative, technical and material support: YL, NL, ZM, ZL, and RY. Study supervision: ZM, RY and MS.
Supporting information
Table S1. The standard formulation of micronutrients and amounts provided in the UNIMMAP
ACKNOWLEDGMENTS
Nan Li was supported by the National Natural Science Foundation of China (81373014), Natural Science Foundation of Beijing Municipality (7194285), the startup funding from the “Incubation” Program of China and Peking University Health Science Center (No. BMU2017YB003) and Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001).
Liu Y, Li N, Mei Z, et al. Effects of prenatal micronutrients supplementation timing on pregnancy‐induced hypertension: Secondary analysis of a double‐blind randomized controlled trial. Matern Child Nutr. 2021;17:e13157. 10.1111/mcn.13157
Yingying Liu and Nan Li contributed equally to this manuscript.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81373014; Natural Science Foundation of Beijing Municipality, Grant/Award Number: 7194285; Young Elite Scientist Sponsorship Program by CAST (YESS), Grant/Award Number: 2018QNRC001; The startup funding from the "Incubation" Program of China and Peking University Health Science Center, Grant/Award Number: No.BMU2017YB003
Contributor Information
Zhiwen Li, Email: lizw@bjmu.edu.cn.
Rongwei Ye, Email: yerw@bjmu.edu.cn.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- Ahsan, T. , Banu, S. , Nahar, Q. , Ahsan, M. , Khan, M. N. , & Islam, S. N. (2013). Serum trace elements levels in preeclampsia and eclampsia: Correlation with the pregnancy disorder. Biological Trace Element Research, 152(3), 327–332. 10.1007/s12011-013-9637-4 [DOI] [PubMed] [Google Scholar]
- Allen, L. H. (2005). Multiple micronutrients in pregnancy and lactation: An overview. The American Journal of Clinical Nutrition, 81(5), 1206S–1212S. 10.1093/ajcn/81.5.1206 [DOI] [PubMed] [Google Scholar]
- Ananth, C. V. , & Basso, O. (2010). Impact of pregnancy‐induced hypertension on stillbirth and neonatal mortality. Epidemiology, 21(1), 118–123. 10.1097/EDE.0b013e3181c297af [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy, L. , Casas, J. P. , Hingorani, A. D. , & Williams, D. J. (2007). Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta‐analysis. BMJ, 335(7627), 974. 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva, E. , & Viteri, F. E. (2003). Iron and oxidative stress in pregnancy. The Journal of Nutrition, 133(5), 1700S–1708S. 10.1093/jn/133.5.1700S [DOI] [PubMed] [Google Scholar]
- Cetin, I. , Berti, C. , & Calabrese, S. (2010). Role of micronutrients in the periconceptional period. Human Reproduction Update, 16(1), 80–95. 10.1093/humupd/dmp025 [DOI] [PubMed] [Google Scholar]
- Chappell, L. C. , Seed, P. T. , Briley, A. L. , Kelly, F. J. , Lee, R. , Hunt, B. J. , … Poston, L. (1999). Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: A randomised trial. Lancet, 354(9181), 810–816. 10.1016/S0140-6736(99)80010-5 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Li, N. , Mei, Z. , Ye, R. , Li, Z. , Liu, J. , & Serdula, M. K. (2018). Micronutrient supplementation during pregnancy and the risk of pregnancy‐induced hypertension: A randomized clinical trial. Clinical Nutrition, 38, 146–151. 10.1016/j.clnu.2018.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde‐Agudelo, A. , Romero, R. , Kusanovic, J. P. , & Hassan, S. S. (2011). Supplementation with Vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. American Journal of Obstetrics and Gynecology, 204(6), 503‐e1. 10.1016/j.ajog.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmugabil, A. , Hamdan, H. Z. , Elsheikh, A. E. , Rayis, D. A. , Adam, I. , & Gasim, G. I. (2016). Serum calcium, magnesium, zinc and copper levels in sudanese women with preeclampsia. PLoS One, 11(12), e0167495. 10.1371/journal.pone.0167495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephraim, R. K. , Osakunor, D. N. , Denkyira, S. W. , Eshun, H. , Amoah, S. , & Anto, E. O. (2014). Serum calcium and magnesium levels in women presenting with pre‐eclampsia and pregnancy‐induced hypertension: a case‐control study in the Cape Coast metropolis, Ghana. BMC Pregnancy and Childbirth, 14, 390. 10.1186/s12884-014-0390-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall, C. H. , Fisher, D. J. , Osmond, C. , Margetts, B. M. , & Maternal Micronutrient Supplementation Study Group . (2009). Multiple micronutrient supplementation during pregnancy in low‐income countries: A meta‐analysis of effects on birth size and length of gestation. Food and Nutrition Bulletin, 30(4 Suppl), S533–S546. 10.1177/15648265090304S408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzl, V. , Flegar‐Mestric, Z. , Perkov, S. , Andrisic, L. , Tatzber, F. , Zarkovic, N. , & Duic, Z. (2013). Trace elements and oxidative stress in hypertensive disorders of pregnancy. Archives of Gynecology and Obstetrics, 287(1), 19–24. 10.1007/s00404-012-2502-4 [DOI] [PubMed] [Google Scholar]
- Haider, B. A. , Yakoob, M. Y. , & Bhutta, Z. A. (2011). Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health, 11(Suppl 3), S19. 10.1186/1471-2458-11-S3-S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L. , Lang, L. , Li, Y. , Liu, Q. , & Yao, Y. (2016). Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy‐induced hypertension and healthy pregnant women: A meta‐analysis. Hypertension in Pregnancy, 35(2), 202–209. 10.3109/10641955.2015.1137584 [DOI] [PubMed] [Google Scholar]
- Henriques, A. C. , Carvalho, F. H. , Feitosa, H. N. , Macena, R. H. , Mota, R. M. , & Alencar, J. C. (2014). Endothelial dysfunction after pregnancy‐induced hypertension. International Journal of Gynaecology and Obstetrics, 124(3), 230–234. 10.1016/j.ijgo.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Jain, S. , Sharma, P. , Kulshreshtha, S. , Mohan, G. , & Singh, S. (2010). The role of calcium, magnesium, and zinc in pre‐eclampsia. Biological Trace Element Research, 133(2), 162–170. 10.1007/s12011-009-8423-9 [DOI] [PubMed] [Google Scholar]
- Jennifer Ribowsky, C. H. (2012). Pregnancy‐induced hypertension. Clinician Reviews, 22(5), 27–32. [Google Scholar]
- Khan, A. I. , Kabir, I. , Ekstrom, E. C. , Asling‐Monemi, K. , Alam, D. S. , Frongillo, E. A. , … Persson, L. A. (2011). Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: A randomized trial in Bangladesh. Nutrition Journal, 10, 134. 10.1186/1475-2891-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Mei, Z. , Zhang, L. , Li, H. , Zhang, Y. , Li, N. , … Serdula, M. K. (2017). Effects of prenatal micronutrient supplementation on spontaneous preterm birth: A double‐blind randomized controlled trial in China. American Journal of Epidemiology, 186(3), 318–325. 10.1093/aje/kwx094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Ye, R. , Zhang, L. , Li, H. , Liu, J. , & Ren, A. (2013). Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension, 61(4), 873–879. 10.1161/HYPERTENSIONAHA.111.00230 [DOI] [PubMed] [Google Scholar]
- Lindheimer, M. D. , Taler, S. J. , & Cunningham, F. G. (2008). Hypertension in pregnancy. Journal of the American Society of Hypertension, 2(6), 484–494. 10.1016/j.jash.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Liu, J. M. , Mei, Z. , Ye, R. , Serdula, M. K. , Ren, A. , & Cogswell, M. E. (2013). Micronutrient supplementation and pregnancy outcomes: Double‐blind randomized controlled trial in China. JAMA Internal Medicine, 173(4), 276–282. 10.1001/jamainternmed.2013.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorzadeh, N. , Kazemirad, Y. , & Kazemirad, N. (2020). Investigating the preventive effect of Vitamins C and E on preeclampsia in nulliparous pregnant women. Journal of Perinatal Medicine, 48(6), 625–629. 10.1515/jpm-2019-0469 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan, U. , Grant, F. K. , Imdad, A. , Bhutta, Z. A. , & Martorell, R. (2013). Effect of multiple micronutrient versus iron‐folate supplementation during pregnancy on intrauterine growth. Nestle Nutrition Institute Workshop Series, 74, 53–62. 10.1159/000348401 [DOI] [PubMed] [Google Scholar]
- Rossi, A. C. , & Mullin, P. M. (2011). Prevention of pre‐eclampsia with low‐dose aspirin or Vitamins C and E in women at high or low risk: A systematic review with meta‐analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 158(1), 9–16. 10.1016/j.ejogrb.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Rumiris, D. , Purwosunu, Y. , Wibowo, N. , Farina, A. , & Sekizawa, A. (2006). Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertension in Pregnancy, 25(3), 241–253. 10.1080/10641950600913016 [DOI] [PubMed] [Google Scholar]
- Sarwar, M. S. , Ahmed, S. , Ullah, M. S. , Kabir, H. , Rahman, G. K. , Hasnat, A. , & Islam, M. S. (2013). Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biological Trace Element Research, 154(1), 14–20. 10.1007/s12011-013-9721-9 [DOI] [PubMed] [Google Scholar]
- Sibai, B. M. (2006). Preeclampsia as a cause of preterm and late preterm (near‐term) births. Seminars in Perinatology, 30(1), 16–19. 10.1053/j.semperi.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Siddiqui, I. A. , Jaleel, A. , Kadri, H. M. , Saeed, W. A. , & Tamimi, W. (2011). Iron status parameters in preeclamptic women. Archives of Gynecology and Obstetrics, 284(3), 587–591. 10.1007/s00404-010-1728-2 [DOI] [PubMed] [Google Scholar]
- Steegers, E. A. P. , von Dadelszen, P. , Duvekot, J. J. , & Pijnenborg, R. (2010). Pre‐eclampsia. The Lancet, 376(9741), 631–644. 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- Tande, D. L. , Ralph, J. L. , Johnson, L. K. , Scheett, A. J. , Hoverson, B. S. , & Anderson, C. M. (2013). First trimester dietary intake, biochemical measures, and subsequent gestational hypertension among nulliparous women. Journal of Midwifery & Women's Health, 58(4), 423–430. 10.1111/jmwh.12007 [DOI] [PubMed] [Google Scholar]
- Toblli, J. E. , Cao, G. , Oliveri, L. , & Angerosa, M. (2012). Effects of iron deficiency anemia and its treatment with iron polymaltose complex in pregnant rats, their fetuses and placentas: oxidative stress markers and pregnancy outcome. Placenta, 33(2), 81–87. 10.1016/j.placenta.2011.11.017 [DOI] [PubMed] [Google Scholar]
- Unicef . (1999). Composition of a multi‐micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. New York.
- Viteri, F. E. , Casanueva, E. , Tolentino, M. C. , Diaz‐Frances, J. , & Erazo, A. B. (2012). Antenatal iron supplements consumed daily produce oxidative stress in contrast to weekly supplementation in Mexican non‐anemic women. Reproductive Toxicology, 34(1), 125–132. 10.1016/j.reprotox.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Wang, I. K. , Muo, C. H. , Chang, Y. C. , Liang, C. C. , Chang, C. T. , Lin, S. Y. , … Morisky, D. E. (2013). Association between hypertensive disorders during pregnancy and end‐stage renal disease: A population‐based study. CMAJ, 185(3), 207–213. 10.1503/cmaj.120230 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The standard formulation of micronutrients and amounts provided in the UNIMMAP
Data Availability Statement
Research data are not shared.


