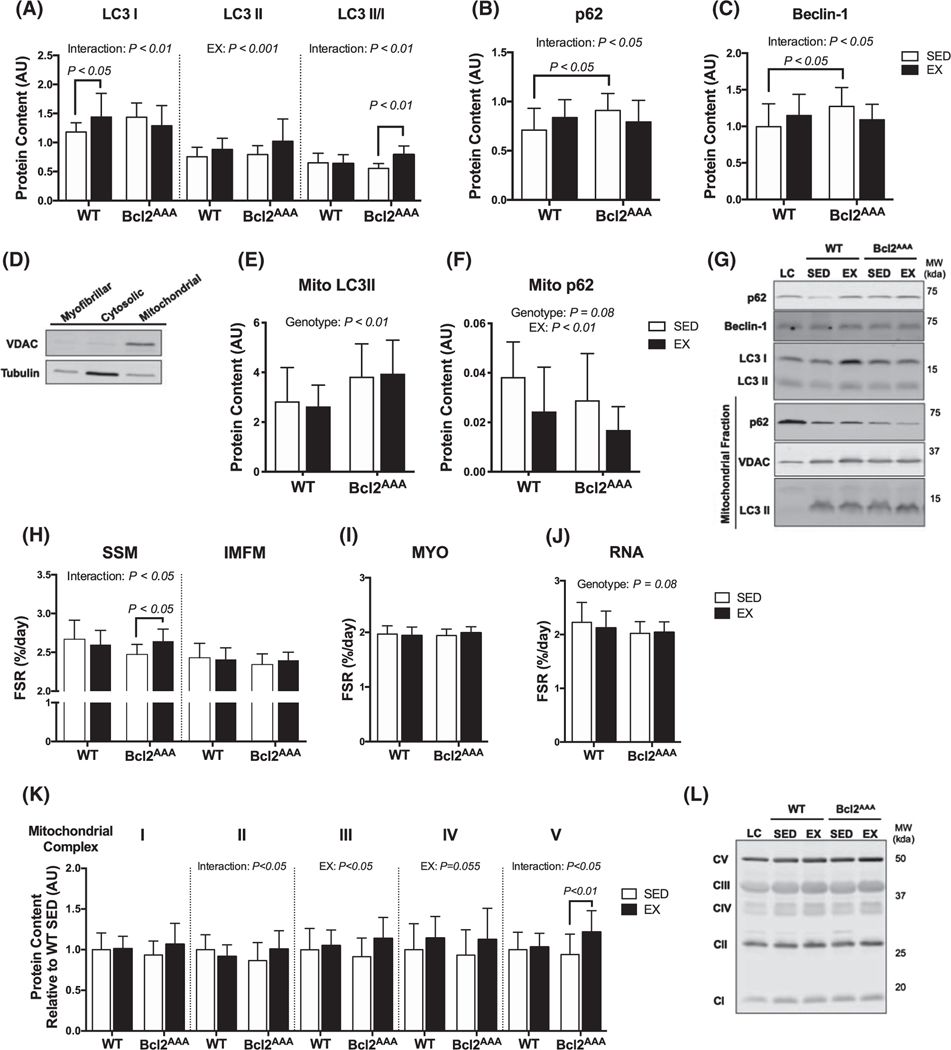
FIGURE 2.
Greater protein turnover contributes to mitochondrial adaptations in autophagy-deficient mice. Quadricep and gastrocnemius muscles were collected after a 4-h fast and 36 h after exercise from wild-type and Bcl2AAA mice that performed 8 wk of exercise training (EX) or remained sedentary (SED). Whole tissue lysates were prepped from quadriceps and mitochondria were isolated from gastrocnemius muscle for immunoblotting. A, Protein content of the autophagy markers LC3I, LC3II, and the ratio of LC3 II/I, B, p62 and (C) Beclin-1 measured with immunoblotting expressed as arbitrary units. D, Demonstration of VDAC and tubulin content in the skeletal muscle fractions. E, Protein content in the mitochondrial fraction of the autophagy markers LC3II and (F) p62 normalized to VDAC content and expressed as arbitrary units. G, Representative blots for (A-F) with loading controls (LC). H, Fractional synthesis rates (%/day) of proteins in the subsarcolemmal (SSM) and intermyofibrillar (IMFM) mitochondrial fractions of quadriceps muscle during 14 d of deuterium oxide labeling. I, Fractional synthesis rates (%/day) of the myofibrillar (MYO) fraction of quadriceps muscle. J, Fractional synthesis rates (%/day) of RNA extracted from quadriceps muscle. K, Protein content of subunits in mitochondrial respiratory complexes I-V measured with immunoblotting expressed in arbitrary units relative to WT SED values. L, Representative blots for (K) with loading control (LC). Full blot images and Ponceau stain of membranes are in supplemental data. Data are mean ± SD. Two-way ANOVA tested effects of exercise and genotype. P values are main effects or post hoc comparisons when significant interaction effects were observed. Protein content data are n = 12–17, FSR data are n = 12–16