Fig. 1. Sugar-induced dILP secretion in Drosophila larvae and adults.
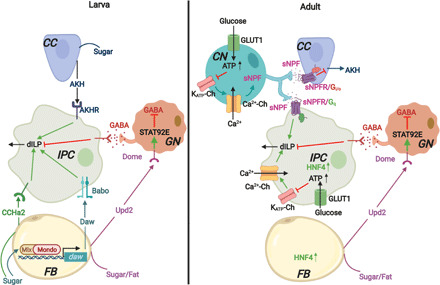
Adult Drosophila IPCs can sense sugar in the hemolymph but larval IPCs cannot. Thus, in larvae, sugar-induced AKH secretion from CC acts through AKHR and indirectly stimulates dILP3 secretion from larval IPCs. Daw, which is transcriptionally induced in the FB by sugar-activated Mondo-Mlx complex, binds the type I (Babo)/type II TGFβ receptor complex on larval IPCs, promoting dILP2 and dILP5 secretion. Likewise, CCHamide-2 (CCHa2), a peptide hormone secreted from the larval FB and midgut (not shown) in response to nutrients including sugar also promotes the expression and secretion of dILPs from IPCs. In contrast, IPCs in adult Drosophila respond to hemolymph glucose directly, through a mechanism analogous to the one described for mammalian pancreatic β cells. GLUT1-mediated glucose uptake in adult IPCs increases glucose catabolism and accompanying mitochondrial ATP production. This leads to the closure of ATP-sensitive potassium channels (KATP-Ch), membrane depolarization, the opening of voltage-dependent Ca2+ channels (Ca2+-Ch), an influx of Ca2+, and subsequent dILP secretion through exocytosis. HNF4, which is highly expressed in adult IPCs, is required for glucose-induced dILP secretion. The CN neuron, which projects to both adult IPCs and AKH-secreting CC with bifurcated axons, responds to hemolymph glucose in a similar fashion in adults. However, Ca2+ influx in these neurons triggers the release of sNPF that binds to sNPF receptors (sNPFR) on IPCs and promotes dILP2 secretion through a Gq-coupled signaling mechanism. This is accompanied by a suppression of AKH secretion from adult CC through an sNPFR/Gi/o-induced signaling cascade. Upd2 secreted from the FB in response to nutrients, including sugar, stimulates Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling in GABAergic neurons (GN) by binding to its receptor Dome and inhibits their firing in a STAT92E-dependent manner. This relieves their inhibitory effect on dILP secretion from both larval and adult IPCs.
