Fig. 2. Convergence of nutrient-sensitive IIS, AKH signaling, and Mondo/Mlx activity on the SIK3/HDAC4/dFOXO axis.
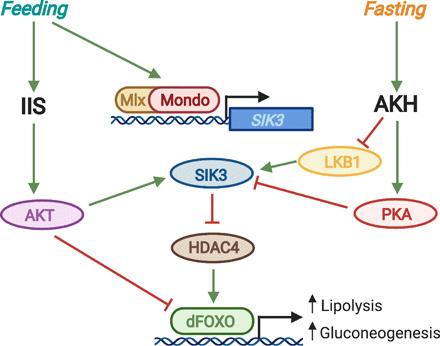
Feeding increases levels of sugar, which binds to and activates the Mondo/Mlx transcription factor complex. The Mondo/Mlx complex then binds to the carbohydrate response element (ChoRE) at the SIK3 promoter and induces SIK3 expression. Feeding also stimulates insulin/insulin-like growth factor signaling (IIS), which leads to AKT activation downstream of the InR/Chico/PI3K cascade. AKT phosphorylates and activates SIK3, which, in turn, phosphorylates and inhibits HDAC4. Active HDAC4 removes inhibitory acetylation on dFOXO and thereby activates it. Thus, SIK3-mediated inhibition of HDAC4 in response to enhanced IIS inhibits dFOXO. In addition, AKT can directly phosphorylate dFOXO to inactivate it and reduce its nuclear localization. dFOXO-induced expression of bmm lipase and PEPCK leads to an increase in lipolysis and gluconeogenesis that eventually elevates sugar levels. Consequently, feeding-mediated IIS activation reduces lipolysis and gluconeogenesis by inhibiting dFOXO. In contrast, fasting stimulates glucagon-like AKH signaling, which leads to PKA activation and inhibition by PKA of SIK3. Moreover, AKH inhibits LKB1 and thereby prevents LKB1-mediated activation of SIK3. As a result, fasting-mediated activation of AKH signaling ultimately leads to dFOXO activation and a subsequent increase in lipolysis and gluconeogenesis.
