Fig. 1.
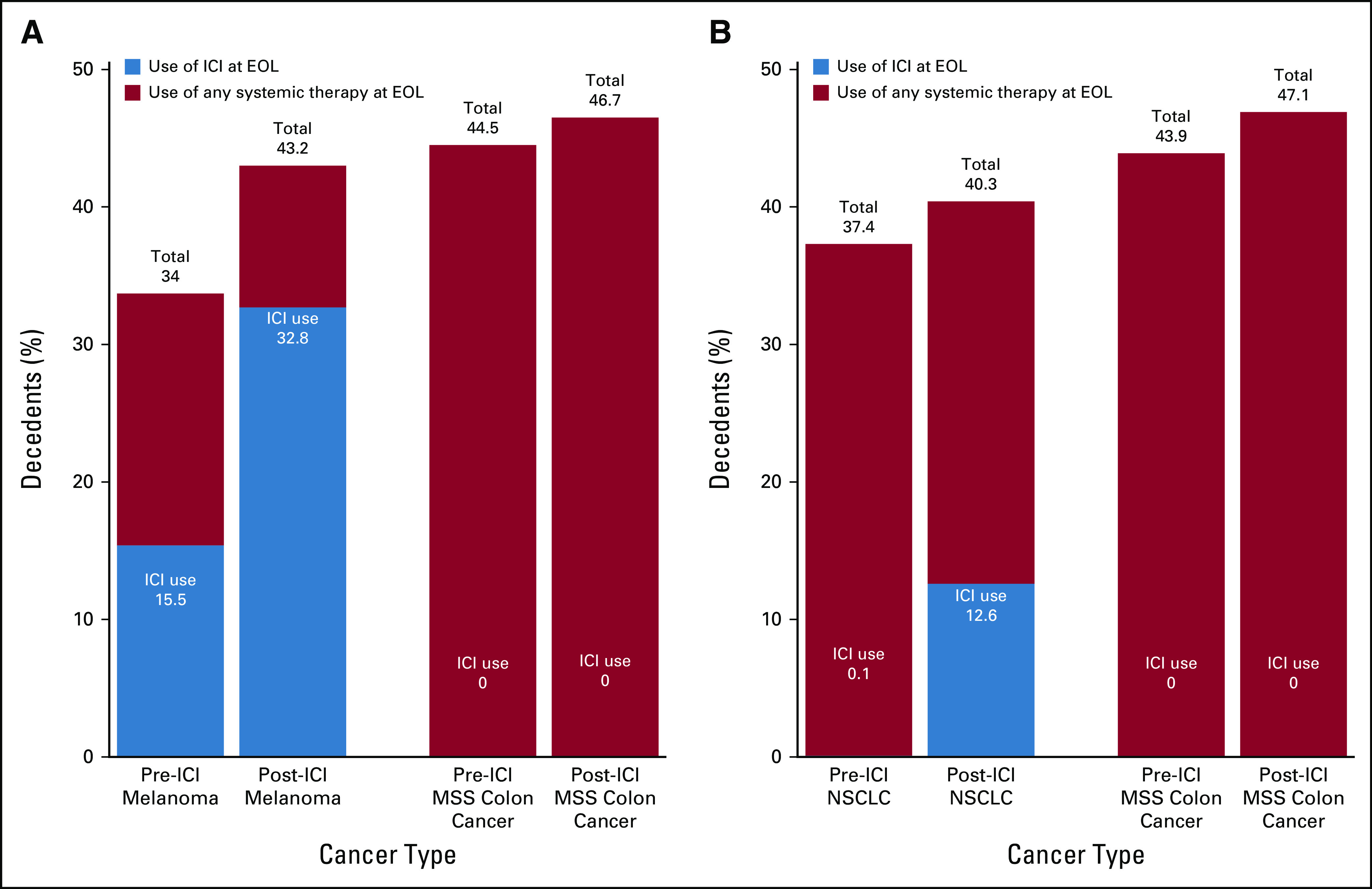
Change in use of immune checkpoint inhibitors (ICIs) and any systemic therapy across pre– and post–Food and Drug Administration (FDA) approval time periods for each cancer type. (A) Depicts the use of ICIs and any systemic therapy at the end of life (EOL) for melanoma and microsatellite stable (MSS) colon cancer before and after the FDA approval of ICIs in melanoma on September 4, 2014. (B) Depicts the use of ICIs and any systemic therapy at EOL for non–small-cell lung cancer (NSCLC) and MSS colon cancer before and after the FDA approval of ICIs for NSCLC on March 4, 2015.
