PURPOSE
Mutations in the HRAS (mHRAS) proto-oncogene occur in 4%-8% of patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Tipifarnib is a farnesyltransferase inhibitor that disrupts HRAS function. We evaluated the efficacy of tipifarnib in patients with R/M mHRAS HNSCC.
METHODS
We enrolled 30 patients with R/M HNSCC in a single-arm, open-label phase II trial of tipifarnib for mHRAS malignancies; one additional patient was treated on an expanded access program. After an ad hoc analysis of the first 16 patients with HNSCC with mHRAS variant allele frequency (VAF) data, enrollment was limited to those with a mHRAS VAF of ≥ 20% (high VAF). The primary end point was objective response rate. Secondary end points included assessing safety and tolerability. Patients received tipifarnib 600 or 900 mg orally twice daily on days 1-7 and 15-21 of 28-day cycles.
RESULTS
Of the 22 patients with HNSCC with high VAF, 20 were evaluable for response at the time of data cutoff. Objective response rate for evaluable patients with high-VAF HNSCC was 55% (95% CI, 31.5 to 76.9). Median progression-free survival on tipifarnib was 5.6 months (95% CI, 3.6 to 16.4) versus 3.6 months (95% CI, 1.3 to 5.2) on last prior therapy. Median overall survival was 15.4 months (95% CI, 7.0 to 29.7). The most frequent treatment-emergent adverse events among the 30 patients with HNSCC were anemia (37%) and lymphopenia (13%).
CONCLUSION
Tipifarnib demonstrated encouraging efficacy in patients with R/M HNSCC with HRAS mutations for whom limited therapeutic options exist (NCT02383927).
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) accounts for more than 500,000 new cancer cases each year worldwide, related primarily to tobacco and alcohol exposure or infection with human papilloma virus (HPV).1 Despite recent advances incorporating programmed death-1 targeting into standard therapy, prognosis remains poor for patients with recurrent and/or metastatic (R/M) HNSCC with an estimated median overall survival of 13-15 months.2 Since the approval of the anti-epidermal growth factor antibody cetuximab more than a decade ago, development of targeted therapies has been stymied by the limited number of druggable targets and the aggressiveness of drug-refractory disease.3,4
CONTEXT
Key Objective
This study evaluated the efficacy of the farnesyltransferase inhibitor tipifarnib in patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) with HRAS mutations, a unique subset of the disease with high unmet needs.
Knowledge Generated
For patients with R/M HNSCC with mutant HRAS variant allele frequency ≥ 20%, tipifarnib treatment produced an objective response rate of 55% and a median overall survival of 15.4 months. The safety profile of tipifarnib was tolerable and manageable in this phase II trial.
Relevance
These results demonstrate encouraging clinical activity with tipifarnib for patients with R/M mutant HRAS HNSCC, warranting further investigation in this patient population.
Activating mutations in the Ras proto-oncogenes (K-, N-, H-) are initiating oncogenic events in human cancer,5 although the development of RAS-targeted therapies has historically been challenging. Farnesyltransferase inhibitors (FTIs) were first evaluated more than 20 years ago as a novel RAS-directed therapy. Mutant RAS must be localized to the plasma membrane to activate downstream signaling, which is dependent upon attachment of a hydrophobic isoprenyl group to its C-terminal tail (prenylation). The predominant form of RAS prenylation is farnesylation, catalyzed by the farnesyltransferase enzyme. It was hypothesized that inhibiting farnesyltransferase would delocalize RAS and inhibit downstream signaling, translating to tumor regressions in RAS-dependent malignancies. Unfortunately, phase II and III clinical trials failed to show significant FTI efficacy against tumor types predicted to be enriched for NRAS and KRAS mutations,5 ending the development of FTIs as a pan–RAS-targeted strategy.
The lack of efficacy in those FTI trials is likely explained by preclinical data demonstrating NRAS and KRAS are susceptible to alternative prenylation events (eg, geranylgeranylation) that maintain membrane localization and pathway activation despite farnesyltransferase inhibition.6 Mutations in the HRAS (mHRAS), however, are uniquely dependent upon farnesylation alone and hence predicted to be particularly susceptible to FTIs.7 In HNSCC patient–derived xenograft models, FTIs induced dramatic regressions in only mHRAS, but not wild-type, models.8 In HNSCC, mHRAS is a driver oncogenic mutation that occurs in approximately 4%-8% of patients9 and defines a predominantly HPV-negative biologic subset characterized by enrichment for wildtype TP53, Caspase-8 mutations, and low copy number alterations.10-13
On the basis of these insights, we developed a clinical trial to revisit FTIs as a therapeutic strategy to target mHRAS in human malignancies. Tipifarnib is a first-in-class nonpeptidomimetic quinolinone that binds and potently inhibits farnesyltransferase (IC50 of 0.86 nM for lamin B farnesylation).14 Its prior clinical development consisted of > 70 clinical studies in solid and hematologic malignancies conducted without genetic selection.15 We developed a phase II trial (KO-TIP-001) to evaluate the objective response rate (ORR) of tipifarnib in patients with incurable mHRAS solid tumors. The interim discovery of a possible efficacy signal for tipifarnib in patients with HNSCC with high mHRAS variant allele frequency (high VAF) led to an amendment to further evaluate tipifarnib in this cohort. This article summarizes our initial experience with tipifarnib as a mHRAS-targeted approach in patients with high–mHRAS VAF HNSCC.
METHODS
Trial Oversight
KO-TIP-001 was an open-label phase II trial approved by the institutional review board or ethics committees at participating institutions. The study was performed in accordance with the Declaration of Helsinki and the International Council for Harmonisation Guidelines on Good Clinical Practice. The study was designed by the sponsor (Kura Oncology) in collaboration with the study investigators. The data analysis and the manuscript were reviewed and approved by the sponsor and the authors.
Patients
Patients with incurable solid tumors harboring missense HRAS mutations were initially enrolled in two cohorts: cohort 1 for thyroid cancer and cohort 2 for nonthyroid solid tumors. Each cohort was individually evaluated with a Simon's 2-stage design allowing stage 2 expansion only if predefined efficacy thresholds were achieved in the first stage. This report focuses on patients with HNSCC enrolled to cohort 2 (Fig 1). After observing two HNSCC responses (of three patients) at the completion of stage 1, cohort 2 was amended to further enroll only patients with mHRAS HNSCC, and cohort 3 was added to evaluate patients with squamous cell carcinoma of other primary sites. Once the cohort 2 primary objective was met with five partial responses (PRs) in nine patients with HNSCC (needed ≥ 4 confirmed objective responses of 18 evaluable), the cohort was expanded to enroll up to 30 patients with mHRAS HNSCC (Fig 1). One additional patient was treated on an expanded access (EA) program using the KO-TIP-001 Protocol (online only). Mutant HRAS status for enrollment was documented by local, approved gene-sequencing platforms; all patients submitted tissue from the most recent tumor biopsy for central laboratory confirmation and VAF determination with the OncoDNA next-generation sequencing (NGS) platform. Specifically, DNAs were extracted from macrodissected tumor cells identified on paraffin-embedded slides, and the HRAS VAF was determined after aligning the reads to a reference genome. The calculated VAF represents the ratio between the number of reads associated with the mutation and the number of reads associated with the wild-type nucleotide, taking into consideration sample heterogeneity. In October 2018, an interim ad hoc analysis of the first 16 patients with HNSCC with available mHRAS VAF data led to a Protocol amendment to limit enrollment to patients with HNSCC with mHRAS VAF of ≥ 20% (Data Supplement, online only). An albumin of ≥ 3.5 g/dL was also required to ensure patients' fitness for therapy except for those whose tumors had mHRAS VAFs of ≥ 35%, a cohort hypothesized to possess particular susceptibility to tipifarnib (Data Supplement). The current analysis only includes those patients with HNSCC meeting these VAF and albumin criteria. A full list of inclusion and exclusion criteria is provided in the Data Supplement. Informed consent for trial participation was obtained from all enrolled patients.
FIG 1.
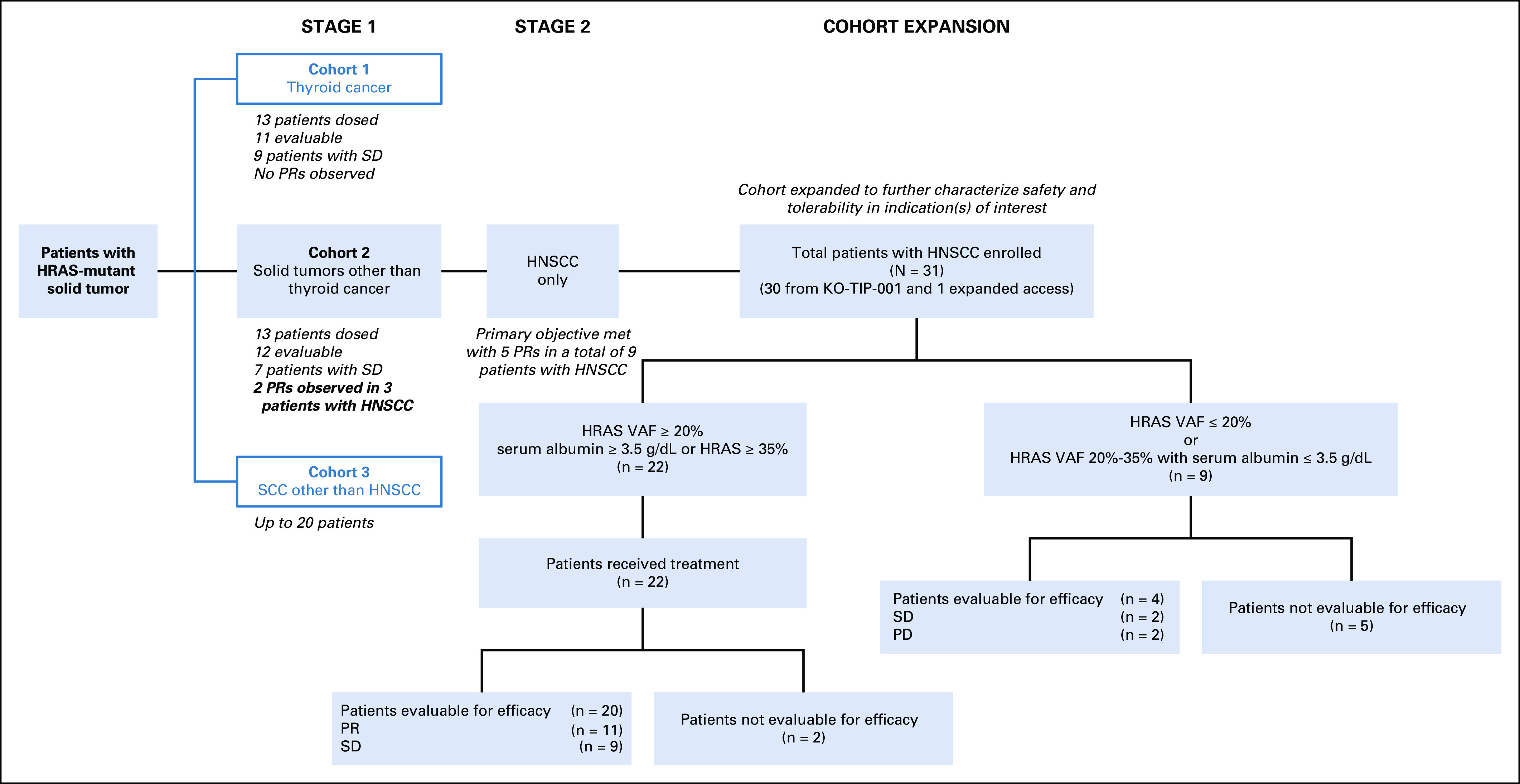
Study overview. HNSCC, head and neck squamous cell carcinoma; PD, progressive disease; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease; VAF, variant allele frequency.
Treatment
Several different dosing schedules for tipifarnib were previously investigated, including low-dose continuous schedules (eg, 300 mg twice daily [twice a day] for 21 days in a 28-day schedule) and high-dose intermittent schedules.16-18 The latter was selected for this study to maximize the potency of farnesyltransferase inhibition achieved. Tipifarnib was initially administered to patients with HNSCC at 900 mg orally twice a day on days 1-7 and 15-21 of 28-day cycles, on the basis of two trials establishing the safety of this schedule.17,18 Of the first 15 patients dosed at 900 mg twice a day, however, nine required dose reduction to manage toxicity (G3 anemia, G3/G1 decreased platelets, G2 peripheral neuropathy, and G2 creatinine increased in two patients each; G2 decreased neutrophil count, G4 decreased WBC count, G3 nausea, G3 hyponatremia, and G3 altered mentality in one patient each), making the median dose of tipifarnib by cycle 2 day 1 for these patients 600 mg twice a day. The Protocol was amended to start tipifarnib at 600 mg twice a day to improve tolerability while helping patients maintain an effective dose for longer duration.
End Points and Assessments
The primary end point was investigator-assessed ORR. Secondary end points included safety and tolerability. Exploratory end points included progression-free survival (PFS), duration of response, OS, and feasibility of molecular analyses using NGS. Radiographic imaging was performed at baseline and approximately every 8 weeks for the first 6 months (cycles 2, 4, and 6) and then every 12 weeks (cycles 9, 12, 15, etc) until disease progression. Adverse events were monitored via clinical and laboratory assessments using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Statistical Analysis
Each cohort followed a Simon's two-stage design in which at least two confirmed PRs were required from the first 11 evaluable patients to proceed to the second stage of seven additional patients. If ≥ 4 responses were observed in 18 patients, tipifarnib treatment would be considered promising. A 30% ORR of interest was assumed. This design had 80% power to detect a difference between 10% and 30% ORR with one-sided significance level of 0.087. Patients were considered evaluable if they had at least one dose of tipifarnib, a baseline tumor scan, and at least one on-treatment scan conducted 6 weeks or more from trial enrollment. Upon rejection of the null hypothesis, the cohort was expanded to allow enrollment of up to 30 patients with mHRAS high-VAF HNSCC with no additional statistical hypotheses tested in the expanded Protocol.
RESULTS
Patient Demographics, Tumor Characteristics, and Tipifarnib Treatment
From September 11, 2015 through April 10, 2020, a total of 31 patients with mHRAS HNSCC from 18 centers in the United States, Europe, and Korea received at least one dose of tipifarnib (30 on the KO-TIP-001 trial and one on an EA program following the KO-TIP-001 Protocol, Fig 1). An ad hoc analysis of the first 16 treated patients with HNSCC performed in October 2018 revealed that efficacy with tipifarnib may be enriched in those with a high VAF—initially defined as ≥ 20% (with five PRs observed among 11 high-VAF patients v 0 of 5 in the low-VAF patients; Data Supplement). The KO-TIP-001 protocol was modified to limit enrollment to patients with HNSCC with a mHRAS VAF of ≥ 20%. An albumin level of ≥ 3.5 g/dL was required for those with a VAF ≥ 20% but < 35% as a marker of overall patient health to best identify those most likely to sustain therapy.19 The albumin criterion was not applied to those whose tumors had a VAF ≥ 35%, a cohort hypothesized to have a high likelihood of tipifarnib benefit based on the ad hoc analysis (Data Supplement). As of April 10, 2020 (data cutoff), 22 of 31 patients with HNSCC treated with tipifarnib met these high VAF and albumin criteria (high-VAF cohort) and are the focus of this analysis. A breakdown of these 22 patients and the other nine patients not included is depicted in Figure 1.
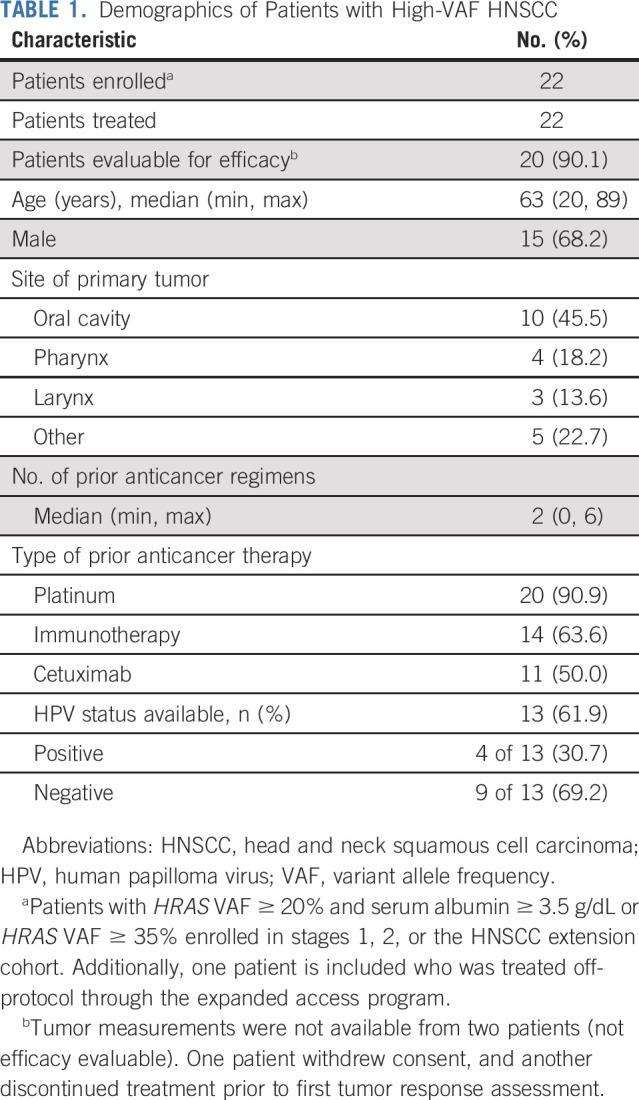
At initial diagnosis, 46% (10 of 22) had oral cavity primary tumors. HPV status was documented by study teams in 13 patients; four (31%) were noted to be HPV-positive (Table 1). Patients had received a median of two prior lines of systemic therapy (range 0-6; one patient received prior radiotherapy only), with all but two having received first-line platinum-based therapy for their locally advanced or metastatic disease: 50% had received cetuximab, 64% had received prior immunotherapy, and 23% both. The median number of treatment cycles initiated was 6.5 (range 1-36). As of data cutoff, tipifarnib treatment continued for three patients.
TABLE 1.
Demographics of Patients with High-VAF HNSCC
Efficacy
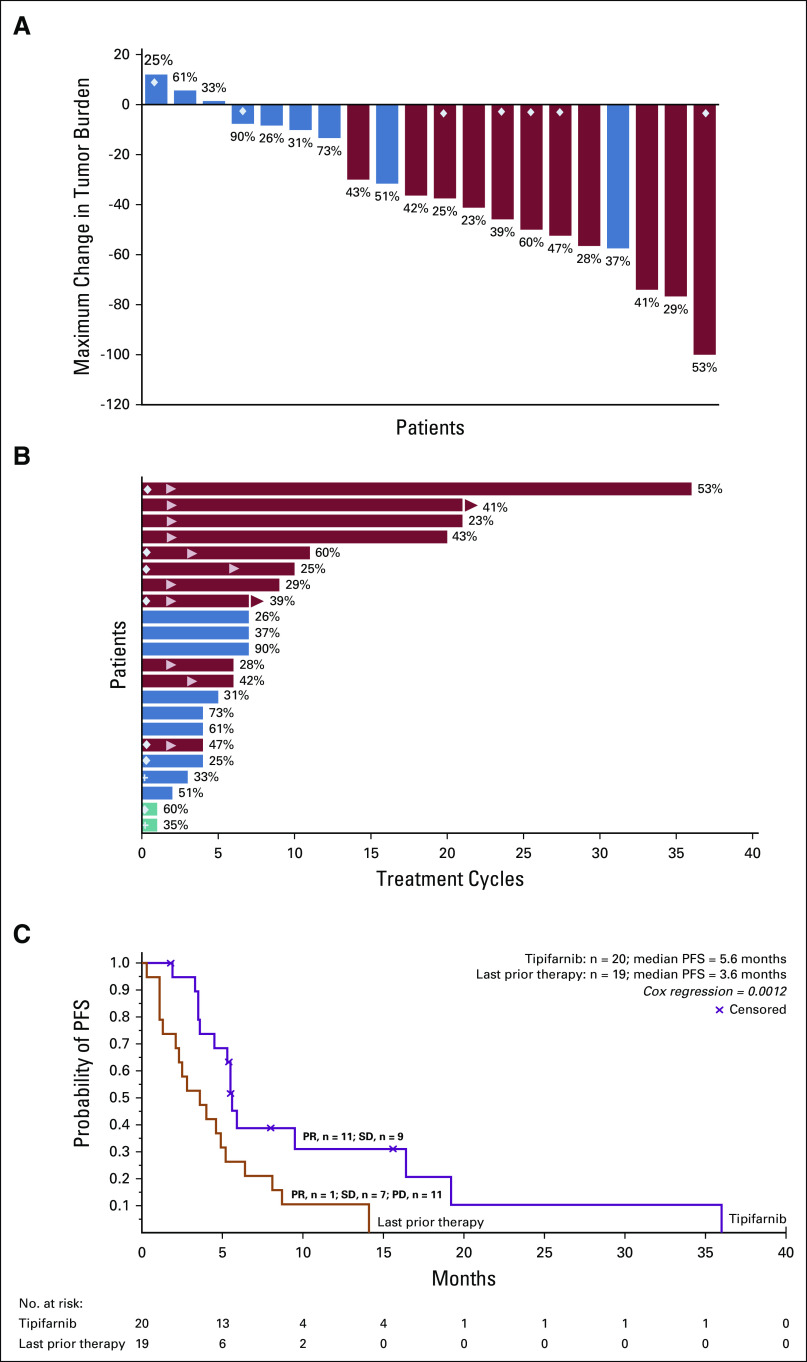
Twenty of the 22 high-VAF treated patients were efficacy evaluable (Table 1); one withdrew consent and another discontinued for symptomatic deterioration prior to efficacy evaluation and were unevaluable. Eleven of the 20 evaluable patients met RECIST v1.1 criteria for confirmed PR (Fig 2A) for an ORR of 55% (11 of 20; 95% CI, 31.5 to 76.9; Table 2). ORR for the intent-to-treat population (n = 22) was 50% (11 of 22; 95% CI, 30.7 to 69.3). Among only trial participants (excluding the EA patient), the ORR was 52.6% (10 of 19; 95% CI, 28.9 to 75.6). Of the 20 evaluable high-VAF patients, 7 of 12 (ORR, 58.3%) patients with a VAF > 35% (range, 37%-90%) had a response compared with 4 of 8 (ORR, 50%) with VAF < 35% (range, 23%-33%). Three of the 12 patients with VAF > 35% had an albumin of < 3.5 g/dL with one (33.3%) achieving response. These responses were achieved rapidly as 8 of 11 met PR criteria at the first tumor assessment (≤ 8 weeks). Five of the six evaluable patients initiated at 600 mg twice a day experienced PRs. Seven of the 11 patients who experienced PRs discontinued treatment because of progressive disease. Of the nine patients who did not experience a response, all had a best response of stable disease (SD), with six achieving minor tumor regression (Fig 2A). Three of the nine patients with SD were on treatment for approximately 7 months. Seven patients with PR remained on therapy for 6 months or longer (Fig 2B). One of the SD patient's target lesions met criteria for a PR, but the overall response was downgraded to SD because of later confirmation of a new, initially equivocal, liver lesion. Response and duration of therapy for all 31 patients with HNSCC treated with tipifarnib are shown in the Data Supplement.
FIG 2.
Efficacy outcomes. Red, PR; blue, SD; green, not evaluable for efficacy; diamond, patient initiated treatment at 600 mg twice a day; cross, patient withdrew consent; arrow in bar, start of response; arrow, active treatment. Numbers at the end of the bars represent VAF for each patient. (A) Maximal change in tumor size. (B) Duration of response to treatment. (C) Kaplan-Meier analysis of PFS. Tick marks indicate censored data. PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; VAF, variant allele frequency.
TABLE 2.
Efficacy Outcomes

Median PFS was statistically significantly improved to 5.6 months (95% CI, 3.6 to 16.4) on tipifarnib compared with 3.6 months (95% CI, 1.3 to 5.2) on last prior therapy (P = .0012 using the Wei-Lin [Cox regression–based] robust estimator)20 (Fig 2C; Table 2). Of the 11 patients with immunotherapy (single agent or in combination) as the last prior line, seven had PR and four had SD on tipifarnib. In those with last prior line other than immunotherapy (n = 8), there were three PRs and four SDs (Data Supplement). As expected, PFS on tipifarnib treatment was higher in patients who experienced a PR (9.5 months; 95% CI, 5.5 to NA; n = 11) than in those who had SD (4.0 months; 95% CI, 1.9 to NA; n = 9). The median OS was 15.4 months (95% CI, 7.0 to 29.7).
Safety
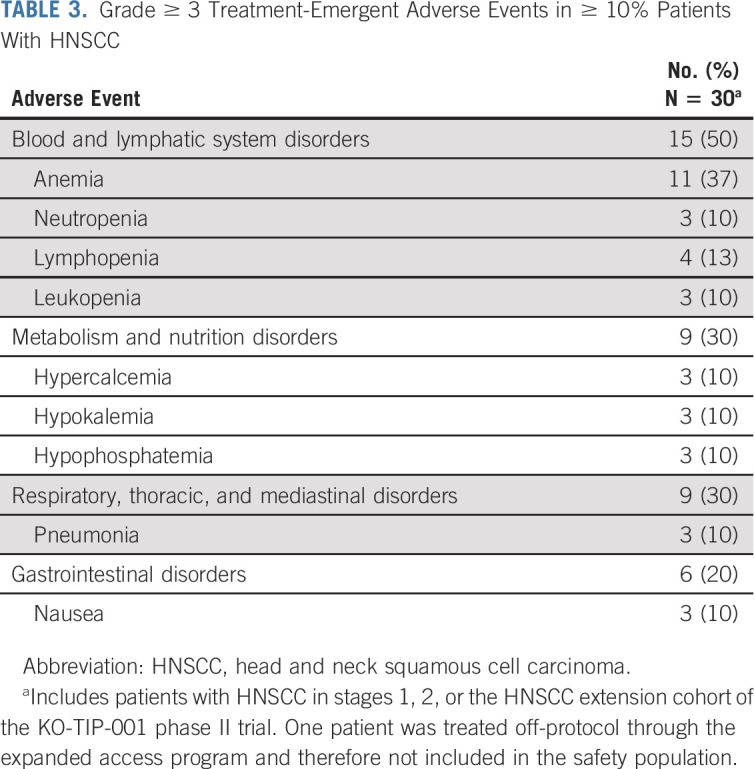
Safety was evaluated in all 30 treated patients with HNSCC (EA patient excluded). Among the most frequently observed treatment emergent adverse events (TEAEs) of grade ≥ 3 in ≥ 10% of patients regardless of VAF or albumin cutoff were hematologic-related events (anemia, neutropenia, leukopenia, and lymphopenia) and GI disturbances (nausea; Table 3). Three patients experienced TEAEs leading to tipifarnib discontinuation: laryngeal obstruction (n =2) and respiratory failure (n = 1). All three events were not related to tipifarnib and possibly related to disease. There were no tipifarnib-related deaths. Adverse events were managed with dose interruption and/or supportive care, including the use of transfusions and growth factors for hematologic events. As of data cutoff, no high-VAF patients have discontinued tipifarnib treatment because of an adverse event. Disease progression was the most common reason for tipifarnib discontinuation.
TABLE 3.
Grade ≥ 3 Treatment-Emergent Adverse Events in ≥ 10% Patients With HNSCC
DISCUSSION
Previous studies reported that approximately 4%-8% of HNSCC tumors are HRAS mutant,21-28 defining a unique HNSCC disease subset that is also characterized by a low frequency of copy number alterations and decreased frequency of TP53 mutations.29 This report describes encouraging antitumor activity with tipifarnib in a heavily pretreated cohort of patients with high–mHRAS VAF (≥ 20%), refractory and/or metastatic HNSCC with an unprecedented ORR of 55% (52.6% in on-trial only patients) as compared with the approximately 15% historical response rate of other standard therapies developed in the platinum-refractory setting, including cetuximab, nivolumab, and pembrolizumab.30 Responses to tipifarnib were rapid and potentially durable, including seven patients with a response longer than 6 months. Importantly, these patients did not experience objective responses with the last prior therapy. The median OS of 15.4 months with tipifarnib is also longer than historically reported for treatments used in a similar setting (5.1-8.4 months).31,32 Another striking observation was the clinical benefit rate for the high–mHRAS VAF efficacy evaluable patients was 100% (11 of 20 with PR and 9 of 20 with SD), providing additional evidence for the role of mHRAS as an oncogenic driver in these tumors and the ability of FTIs to therapeutically target it. To our knowledge, this is the first study hypothesizing an association between the efficacy of a molecularly targeted therapy and the VAF of the hypothesized biologic target. Although the sample size was small, the strategy of limiting enrollment to those with disease where mHRAS is most likely a clonal, oncogenic driver ensured the most rigorous evaluation of FTI effectiveness in this biologic context. VAF as a predictive biomarker for tipifarnib efficacy, however, will require further evaluation.
The efficacy of targeting HRAS mutations may also be influenced by cellular lineage as tipifarnib activity observed in mHRAS salivary cancers33 (8% ORR) and urothelial carcinomas34 (29% ORR) differs from the HNSCC signal. Distinct genomic contexts, biochemical consequences of inhibiting HRAS signaling, and contribution of other farnesylated targets may be factors modifying tipifarnib outcomes among different tumor types.
Although mHRAS remains a rare HNSCC genomic subset, clinical resistance to cetuximab in patients with advanced HRAS wild-type HNSCC may be associated with the emergence of HRAS mutations,35 consistent with the role RAS activation plays in mediating cetuximab resistance in colorectal cancer.36 This suggests that the frequency of HRAS mutations observed with genomic profiling may be dependent upon the clinical setting in which it is performed and that tipifarnib could be a novel approach to prevent or treat cetuximab drug resistance in HNSCC. With emerging tumor-agnostic indications for molecularly targeted and immunotherapy approaches that require NGS analysis, we anticipate that genomic characterization of HNSCC tumors will continue to expand and provide greater insight into clinical settings that enrich for mHRAS.
Tipifarnib was well-tolerated overall in patients with HNSCC with a TEAE profile consistent with the previously reported safety profile of tipifarnib. The mechanistic basis of tipifarnib toxicity is not well-understood but may be related to the recent discovery of tipifarnib as an inhibitor of the CXCL12/CXCR4 pathway.37 CXCL12 is a cytokine that is essential for the maturation of neutrophils, production of platelets, and homing of lymphocytes, among other functions.38,39 Further research on the effects of tipifarnib on the CXCL12 pathway and the genetic variability of this chemokine among patients could contribute to a better understanding of predicting tipifarnib toxicity and how it might be combined with current or future immunotherapeutic approaches.
The initial FTI development effort made more than 20 years ago and the strategies used in this study illustrate the complexities of developing targeted therapies beyond simply matching the right drug to the appropriate molecular target. For tipifarnib, the meaningful efficacy signal in patients with treatment-refractory mHRAS HNSCC was discovered only after (1) revisiting the FTI class with a trial design focused on testing the unique vulnerability of mHRAS disease, (2) the recognition that confirmation of the HNSCC signal would be most efficiently accomplished by enriching for higher VAF in enrollment, and (3) changing the tipifarnib dose to improve tolerability. What still remain to be understood are the lineage-specific effects of tipifarnib among different mHRAS cancers, validating that high mHRAS VAF is requisite for tipifarnib benefit, the molecular mechanisms of acquired resistance, and how rational combinations may expand the utility of FTI inhibition to other settings (eg, any VAF setting, non-HNSCC tumors, and HRAS amplified or overexpressed). The main caveats of this report include the nonrandomized, open-label design and the small sample size for the analysis. Nonetheless, the efficacy signal observed is impressive for a targeted therapy in a biomarker-selected HNSCC patient cohort and supports continued investigation of tipifarnib. To this end, a pivotal study (AIM-HN and SEQ-HN Study, NCT03719690) evaluating the efficacy and safety of tipifarnib in mHRAS HNSCC (AIM-HN) and the impact of HRAS mutations on HNSCC therapies (SEQ-HN) is currently ongoing. For AIM-HN, patients with mHRAS HNSCC regardless of VAF will be enrolled to further evaluate tipifarnib efficacy and better define the role of VAF as a predictive biomarker of benefit.
ACKNOWLEDGMENT
The authors would like to thank Jeanne Britt and Vishnu Mishra for valuable contributions to the trial. Medical writing and editorial support for the preparation of this manuscript was provided by Tamar Aprahamian, PhD, of JetPub Scientific Communications and was funded by Kura Oncology, San Diego, CA, in accordance with Good Publication Practice (GPP3) guidelines.
APPENDIX 1
Belgium
Laurence Faugeras, Jean-Pascal Machiels, Pol Specenier
France
Caroline Even, Jérôme Fayette, Antoine Italiano, Esma Saada-Bouzid
Germany
Stephan Hackenberg
Greece
Amanda Psyrri
Italy
Lisa Licitra
Korea
Myung-Ju Ahn, Sung-Bae Kim
Netherlands
Sjoukje Oosting
Spain
Marta Guix Arnau, Virginia Arrazubi Arrula, Valentina Boni, Miguel Pastor Borgonon, Beatriz Castelo, Enriqueta Felip, Juan J. Grau, Maria Pilar Lopez-Criado, Maria José Flor Oncala, Lara Iglesias, Jose M. Trigo Perez
United Kingdom
Martin Forster, Kevin Harrington, Ioanna Nixon
United States of America
Jessica Bauman, Keith C. Bible, Maria E. Cabanillas, Nicole Chau, Robert Haddad, Alan Ho, David Hong, Hyunseok Kang, Ranee Mehra, Mohammad Razaq, Nabil F Saba, Deborah Wong, Francis Worden
Alan L. Ho
Consulting or Advisory Role: Bristol-Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Rgenta, Exelixis, Genentech/Roche, Affyimmune
Speakers' Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche, AstraZeneca, Bayer, Kura Oncology, Kolltan Pharmaceuticals, Eisai, Bristol-Myers Squibb, Astellas Pharma, Novartis, Merck, Pfizer, Ayala Pharmaceuticals, Allos Therapeutics, Daiichi Sankyo, Elevar Therapeutics
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, Klus Pharma
Irene Brana
Consulting or Advisory Role: Merck Sharp and Dohme, Rakuten Medical, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now
Speakers' Bureau: Bristol-Myers Squibb, Merck Serono, Roche
Research Funding: AstraZeneca, Bristol-Myers Squibb, Celgene, Gliknik, GlaxoSmithKline, Janssen Oncology, Kura Oncology, Merck Sharp and Dohme, Novartis, Orion Pharma GmbH, Pfizer, Roche, Shattuck Labs, Nanobiotix, Seattle Genetics
Travel, Accommodations, Expenses: AstraZeneca Spain, Merck Serono
Robert Haddad
Employment: Dana-Farber Cancer Institute
Leadership: NCCN
Consulting or Advisory Role: Celgene, Merck, Eisai, Bristol-Myers Squibb, AstraZeneca, Pfizer, Loxo, Genentech, Immunomic Therapeutics, GlaxoSmithKline, Gilead Sciences, Vaccinex, EMD Serono, BioNTech AG, Achilles Therapeutics
Research Funding: Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Celgene, AstraZeneca, VentiRx, Genentech, Pfizer, Kura Oncology
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Nanobiotix, ISA Pharmaceuticals
Jessica Bauman
Consulting or Advisory Role: Pfizer, Bayer, AstraZeneca, Kura Oncology
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Trident Pharmaceuticals
Sjoukje Oosting
Research Funding: Celldex
Deborah J. Wong
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, Sanofi/Aventis, Blueprint Medicines
Research Funding: BioMed Valley Discoveries, Merck Serono, Merck Sharp and Dohme, ARMO BioSciences, AstraZeneca/MedImmune, Kura Oncology, Regeneron, Genentech/Roche, Bristol-Myers Squibb, FSTAR, Pfizer, Astellas Pharma, Enzychem Lifesciences, Lilly, Elevar Therapeutics
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, TAKEDA
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly, MSD, TAKEDA, Alpha pharmaceutical
Caroline Even
Consulting or Advisory Role: Innate Pharma, Bristol-Myers Squibb, MSD Oncology, Merck Serono
Jerome Fayette
Honoraria: AstraZeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, Merck Serono, Innate Pharma, Roche
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, Merck Serono, Innate Pharma, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Merck Sharp and Dohme
Kevin Harrington
Honoraria: Merck Sharp and Dohme, Amgen, Merck Serono, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Replimune, Oncolys BioPharma, Mersana
Consulting or Advisory Role: Merck Sharp and Dohme, Merck Serono, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Replimune
Speakers' Bureau: Merck Sharp and Dohme, Merck Serono, Bristol-Myers Squibb
Research Funding: AstraZeneca, Merck Sharp and Dohme, Boehringer Ingelheim, Replimune
David S. Hong
Stock and Other Ownership Interests: OncoResponse, Telperian
Consulting or Advisory Role: Bayer, Guidepoint Global, GLG, Alpha Insights, Axiom Biotechnologies, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, Trieza Therapeutics, WebMD, Infinity Pharmaceuticals, Amgen, Adaptimmune, Boxer Capital, ECOR1, Tavistock, Baxter, COG, Genentech, Group H, Janssen, Acuta, HCW Precision, Prime Oncology, ST Cube, Alkermes, Aumbiosciences, Antheneum, Barclays, Bridgebio, CDR-Life AG, Cor2Ed, Gilead, Immunogen, Liberium, Oncologia Brasil, Pharma Intelligence, POET Congress, Turning Point Therapeutics, Ziopharm
Research Funding: Genentech (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Adaptimmune (Inst), Abbvie (Inst), Bayer (Inst), Infinity Pharmaceuticals (Inst), Kite Pharma (Inst), MedImmune (Inst), NCI-CTEP (Inst), Fate Therapeutics (Inst), Pfizer (Inst), Novartis (Inst), Numab (Inst), Turning Point Therapeutics (Inst), Verstatem (Inst), Kyowa (Inst), Loxo (Inst), Merck (Inst), Eisai (Inst), Genmab (Inst), Mirati (Inst), Mologen (Inst), Takeda (Inst), AstraZeneca (Inst), Navier (Inst), VM Oncology (Inst), Erasca, Inc. (Inst), Bristol Myers Squibb (Inst), Adlai Nortye (Inst), Seagen (Inst), Deciphera (Inst), Pyramid Biosciences (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Genmab, SITC, Bayer Schering Pharma, ASCO, AACR, Telperian
Sung-Bae Kim
Honoraria: DAEHWA Pharmaceutical, ISU ABXIS
Consulting or Advisory Role: Lilly, AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis
Research Funding: Novartis, Dongkook Pharma, Genzyme
Lisa Licitra
Consulting or Advisory Role: Eisai, Boehringer Ingelheim, AstraZeneca, SOBI, Novartis, Bayer, MSD, Merck Serono, Roche, Bristol-Myers Squibb, Incyte, Doxapharma, GlaxoSmithKline, Nanobiotix, Debiopharm Group, Amgen, Ipsen
Research Funding: AstraZeneca, Novartis, Roche, MSD, Eisai, Merck Serono, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Exelixis, IRX Therapeutics, Medpace, Pfizer, Debiopharm Group, Roche
Travel, Accommodations, Expenses: Merck Serono, Bayer, Bristol-Myers Squibb, MSD, Eisai, AstraZeneca
Nabil F. Saba
Honoraria: Merck, Lilly, Pfizer, Bristol-Myers Squibb, CUE Biopharma, GlaxoSmithKline, Aduro Biotech, Kura Oncology, Genentech/Roche
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Merck, Lilly, Bluprint, Biontech
Research Funding: Bristol-Myers Squibb, Exelixis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck, Pfizer, Lilly, GlaxoSmithKline, Genentech/Roche, Bluprint
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol-Myers Squibb, Bayer, Regeneron
Consulting or Advisory Role: Merck, Loxo, Bristol-Myers Squibb, Eisai, Bayer, CUE Biopharma, Rakuten Medical, Regeneron
Research Funding: Pfizer, Merck, Eisai, Bristol-Myers Squibb, Loxo, Oragenics, Lilly
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer
Binaifer Balsara
Employment: Kura Oncology
Stock and Other Ownership Interests: Kura Oncology
Mollie Leoni
Employment: Kura Oncology, Kyowa Kirin International
Stock and Other Ownership Interests: Kura Oncology
Travel, Accommodations, Expenses: Kura Oncology, Kyowa Kirin International
Catherine Scholz
Employment: Kura Oncology, H3 Biomedicine
Stock and Other Ownership Interests: Kura Oncology
Patents, Royalties, Other Intellectual Property: Methods of Treating Cancer Patients With Farnesyltransferase Inhibitors (FTI treatment of H-Ras mutant cancers), co-Inventor (014168-0011-999), Methods of Treating Cancer With Farnesyltransferase Inhibitors (FTI treatment of a CXCL12-expressing cancer), co-Inventor (014168-0021-999), Methods of Treating Cancer Patients With Farnesyltransferase Inhibitors (FTI treatment of Casitas B cell lymphoma [CBL] mutant cancers), co-Inventor (014168-0024-228), Therapies For Squamous Cell Carcinomas (FTI treatment of SCC with high frequencies of H-Ras mutant allele frequency), co-Inventor (014168-0051-888)
Travel, Accommodations, Expenses: Kura Oncology
No other potential conflicts of interest were reported.
See accompanying editorial on page 1839
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology Virtual Annual Meeting, May 29-June 1, 2020.
SUPPORT
Supported by research funding from Kura Oncology. The corresponding author A.L.H. was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and the Geoffrey Beene Cancer Research Center at Memorial Sloan Kettering Cancer Center.
CLINICAL TRIAL INFORMATION
NCT02383927 (KO-TIP-001)
DATA SHARING STATEMENT
Kura Oncology agrees to share individual participant data that underlie the results reported in this article (after deidentification), including the study Protocol and statistical analysis plan. Data availability will begin 9 months after publication and will be available 36 months after publication. To gain access, data requestors should submit a proposal to medicalaffairs@kuraoncology.com. Proposals will be reviewed by an independent review committee identified for this purpose.
AUTHOR CONTRIBUTIONS
Conception and design: Alan L. Ho, Irene Brana, Valentina Boni, Robert Haddad, Pol Specenier, Bridget Martell, Antonio Gualberto
Financial support: Bridget Martell
Administrative support: Bridget Martell
Provision of study materials or patients: Alan L. Ho, Robert Haddad, Keith Bible, Myung-Ju Ahn, Valentina Boni, Caroline Even, Maria José Flor, Kevin Harrington, Bridget Martell
Collection and assembly of data: Alan L. Ho, Irene Brana, Jessica Bauman, Keith Bible, Sjoukje Oosting, Deborah J. Wong, Caroline Even, Jerome Fayette, Maria José Flor, Kevin Harrington, David S. Hong, Ioanna Nixon, Nabil F. Saba, Stephan Hackenberg, Pol Specenier, Binaifer Balsara, Mollie Leoni, Bridget Martell, Antonio Gualberto
Data analysis and interpretation: Alan L. Ho, Irene Brana, Robert Haddad, Jessica Bauman, Deborah J. Wong, Myung-Ju Ahn, Valentina Boni, Kevin Harrington, David S. Hong, Sung-Bae Kim, Lisa Licitra, Ioanna Nixon, Francis Worden, Binaifer Balsara, Mollie Leoni, Bridget Martell, Catherine Scholz, Antonio Gualberto
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tipifarnib in Head and Neck Squamous Cell Carcinoma With HRAS Mutations
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alan L. Ho
Consulting or Advisory Role: Bristol-Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Rgenta, Exelixis, Genentech/Roche, Affyimmune
Speakers' Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche, AstraZeneca, Bayer, Kura Oncology, Kolltan Pharmaceuticals, Eisai, Bristol-Myers Squibb, Astellas Pharma, Novartis, Merck, Pfizer, Ayala Pharmaceuticals, Allos Therapeutics, Daiichi Sankyo, Elevar Therapeutics
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, Klus Pharma
Irene Brana
Consulting or Advisory Role: Merck Sharp and Dohme, Rakuten Medical, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now
Speakers' Bureau: Bristol-Myers Squibb, Merck Serono, Roche
Research Funding: AstraZeneca, Bristol-Myers Squibb, Celgene, Gliknik, GlaxoSmithKline, Janssen Oncology, Kura Oncology, Merck Sharp and Dohme, Novartis, Orion Pharma GmbH, Pfizer, Roche, Shattuck Labs, Nanobiotix, Seattle Genetics
Travel, Accommodations, Expenses: AstraZeneca Spain, Merck Serono
Robert Haddad
Employment: Dana-Farber Cancer Institute
Leadership: NCCN
Consulting or Advisory Role: Celgene, Merck, Eisai, Bristol-Myers Squibb, AstraZeneca, Pfizer, Loxo, Genentech, Immunomic Therapeutics, GlaxoSmithKline, Gilead Sciences, Vaccinex, EMD Serono, BioNTech AG, Achilles Therapeutics
Research Funding: Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Celgene, AstraZeneca, VentiRx, Genentech, Pfizer, Kura Oncology
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Nanobiotix, ISA Pharmaceuticals
Jessica Bauman
Consulting or Advisory Role: Pfizer, Bayer, AstraZeneca, Kura Oncology
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Trident Pharmaceuticals
Sjoukje Oosting
Research Funding: Celldex
Deborah J. Wong
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, Sanofi/Aventis, Blueprint Medicines
Research Funding: BioMed Valley Discoveries, Merck Serono, Merck Sharp and Dohme, ARMO BioSciences, AstraZeneca/MedImmune, Kura Oncology, Regeneron, Genentech/Roche, Bristol-Myers Squibb, FSTAR, Pfizer, Astellas Pharma, Enzychem Lifesciences, Lilly, Elevar Therapeutics
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, TAKEDA
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly, MSD, TAKEDA, Alpha pharmaceutical
Caroline Even
Consulting or Advisory Role: Innate Pharma, Bristol-Myers Squibb, MSD Oncology, Merck Serono
Jerome Fayette
Honoraria: AstraZeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, Merck Serono, Innate Pharma, Roche
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, Merck Serono, Innate Pharma, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Merck Sharp and Dohme
Kevin Harrington
Honoraria: Merck Sharp and Dohme, Amgen, Merck Serono, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Replimune, Oncolys BioPharma, Mersana
Consulting or Advisory Role: Merck Sharp and Dohme, Merck Serono, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Replimune
Speakers' Bureau: Merck Sharp and Dohme, Merck Serono, Bristol-Myers Squibb
Research Funding: AstraZeneca, Merck Sharp and Dohme, Boehringer Ingelheim, Replimune
David S. Hong
Stock and Other Ownership Interests: OncoResponse, Telperian
Consulting or Advisory Role: Bayer, Guidepoint Global, GLG, Alpha Insights, Axiom Biotechnologies, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, Trieza Therapeutics, WebMD, Infinity Pharmaceuticals, Amgen, Adaptimmune, Boxer Capital, ECOR1, Tavistock, Baxter, COG, Genentech, Group H, Janssen, Acuta, HCW Precision, Prime Oncology, ST Cube, Alkermes, Aumbiosciences, Antheneum, Barclays, Bridgebio, CDR-Life AG, Cor2Ed, Gilead, Immunogen, Liberium, Oncologia Brasil, Pharma Intelligence, POET Congress, Turning Point Therapeutics, Ziopharm
Research Funding: Genentech (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Adaptimmune (Inst), Abbvie (Inst), Bayer (Inst), Infinity Pharmaceuticals (Inst), Kite Pharma (Inst), MedImmune (Inst), NCI-CTEP (Inst), Fate Therapeutics (Inst), Pfizer (Inst), Novartis (Inst), Numab (Inst), Turning Point Therapeutics (Inst), Verstatem (Inst), Kyowa (Inst), Loxo (Inst), Merck (Inst), Eisai (Inst), Genmab (Inst), Mirati (Inst), Mologen (Inst), Takeda (Inst), AstraZeneca (Inst), Navier (Inst), VM Oncology (Inst), Erasca, Inc. (Inst), Bristol Myers Squibb (Inst), Adlai Nortye (Inst), Seagen (Inst), Deciphera (Inst), Pyramid Biosciences (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Genmab, SITC, Bayer Schering Pharma, ASCO, AACR, Telperian
Sung-Bae Kim
Honoraria: DAEHWA Pharmaceutical, ISU ABXIS
Consulting or Advisory Role: Lilly, AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis
Research Funding: Novartis, Dongkook Pharma, Genzyme
Lisa Licitra
Consulting or Advisory Role: Eisai, Boehringer Ingelheim, AstraZeneca, SOBI, Novartis, Bayer, MSD, Merck Serono, Roche, Bristol-Myers Squibb, Incyte, Doxapharma, GlaxoSmithKline, Nanobiotix, Debiopharm Group, Amgen, Ipsen
Research Funding: AstraZeneca, Novartis, Roche, MSD, Eisai, Merck Serono, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Exelixis, IRX Therapeutics, Medpace, Pfizer, Debiopharm Group, Roche
Travel, Accommodations, Expenses: Merck Serono, Bayer, Bristol-Myers Squibb, MSD, Eisai, AstraZeneca
Nabil F. Saba
Honoraria: Merck, Lilly, Pfizer, Bristol-Myers Squibb, CUE Biopharma, GlaxoSmithKline, Aduro Biotech, Kura Oncology, Genentech/Roche
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Merck, Lilly, Bluprint, Biontech
Research Funding: Bristol-Myers Squibb, Exelixis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck, Pfizer, Lilly, GlaxoSmithKline, Genentech/Roche, Bluprint
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol-Myers Squibb, Bayer, Regeneron
Consulting or Advisory Role: Merck, Loxo, Bristol-Myers Squibb, Eisai, Bayer, CUE Biopharma, Rakuten Medical, Regeneron
Research Funding: Pfizer, Merck, Eisai, Bristol-Myers Squibb, Loxo, Oragenics, Lilly
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer
Binaifer Balsara
Employment: Kura Oncology
Stock and Other Ownership Interests: Kura Oncology
Mollie Leoni
Employment: Kura Oncology, Kyowa Kirin International
Stock and Other Ownership Interests: Kura Oncology
Travel, Accommodations, Expenses: Kura Oncology, Kyowa Kirin International
Catherine Scholz
Employment: Kura Oncology, H3 Biomedicine
Stock and Other Ownership Interests: Kura Oncology
Patents, Royalties, Other Intellectual Property: Methods of Treating Cancer Patients With Farnesyltransferase Inhibitors (FTI treatment of H-Ras mutant cancers), co-Inventor (014168-0011-999), Methods of Treating Cancer With Farnesyltransferase Inhibitors (FTI treatment of a CXCL12-expressing cancer), co-Inventor (014168-0021-999), Methods of Treating Cancer Patients With Farnesyltransferase Inhibitors (FTI treatment of Casitas B cell lymphoma [CBL] mutant cancers), co-Inventor (014168-0024-228), Therapies For Squamous Cell Carcinomas (FTI treatment of SCC with high frequencies of H-Ras mutant allele frequency), co-Inventor (014168-0051-888)
Travel, Accommodations, Expenses: Kura Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sklan A, Collingridge D: Treating head and neck cancer: For better or for worse? Lancet Oncol 18:570-571, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Burtness B Harrington KJ Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Cohen EEW Licitra LF Burtness B, et al. : Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol 28:2526-2532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galot R Le Tourneau C Guigay J, et al. : Personalized biomarker-based treatment strategy for patients with squamous cell carcinoma of the head and neck: EORTC position and approach. Ann Oncol 29:2313-2327, 2018; [DOI] [PubMed] [Google Scholar]
- 5.Cox AD, Der CJ: Ras history: The saga continues. Small GTPases 1:2-27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karp JE Kaufmann SH Adjei AA, et al. : Current status of clinical trials of farnesyltransferase inhibitors. Curr Opin Oncol 13:470-476, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Untch BR Dos Anjos VC Garcia-Rendueles MER, et al. : Tipifarnib Inhibits HRAS-Driven dedifferentiated thyroid cancers. Cancer Res 78:4642-4657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilardi M Wang Z Proietto M, et al. : Tipifarnib as a precision therapy for HRAS-mutant head and neck squamous cell carcinomas. Mol Cancer Ther 19:1784-1796, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mountzios G, Rampias T, Psyrri A: The mutational spectrum of squamous-cell carcinoma of the head and neck: Targetable genetic events and clinical impact. Ann Oncol 25:1889-1900, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Stransky N Egloff AM Tward AD, et al. : The mutational landscape of head and neck squamous cell carcinoma. Science 333:1157-1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Network : Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576-582, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickering CR Zhang J Yoo SY, et al. : Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov 3:770-781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leemans CR, Snijders PJF, Brakenhoff RH: The molecular landscape of head and neck cancer. Nat Rev Cancer 18:269-282, 2018 [DOI] [PubMed] [Google Scholar]
- 14.End DW Smets G Todd AV, et al. : Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res 61:131-137, 2001 [PubMed] [Google Scholar]
- 15.Appels NMGM: Development of farnesyl transferase inhibitors: A review. Oncologist 10:565-578, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Zujewski J Horak ID Bol CJ, et al. : Phase I and pharmacokinetic study of farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol 18:927-941, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Kirschbaum MH Synold T Stein AS, et al. : A phase 1 trial dose-escalation study of tipifarnib on a week-on, week-off schedule in relapsed, refractory or high-risk myeloid leukemia. Leukemia 25:1543-1547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lara PN Law LY Wright JJ, et al. : Intermittent dosing of the farnesyl transferase inhibitor tipifarnib (R115777) in advanced malignant solid tumors: A phase I California cancer consortium trial. Anticancer Drugs 16:317-321, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hannan JL, Radwany SM, Albanese T: In-hospital mortality in patients older than 60 years with very low albumin levels. J Pain Symptom Manage 43:631-637, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ: The robust inference for the cox proportional hazards model. J Am Stat Assoc 84:1074, 1989 [Google Scholar]
- 21.Agrawal N Frederick MJ Pickering CR, et al. : Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333:1154-1157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braig F Voigtlaender M Schieferdecker A, et al. : Liquid biopsy monitoring uncovers acquired RAS-mediated resistance to cetuximab in a substantial proportion of patients with head and neck squamous cell carcinoma. Oncotarget 7:42988-42995, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canning M Guo G Yu M, et al. : Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front Cell Dev Biol 7:52, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer JD Burtness B Le QT, et al. : The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol 16:669-683, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Lyu H Li M Jiang Z, et al. : Correlate the TP53 mutation and the HRAS mutation with immune signatures in head and neck squamous cell cancer. Comput Struct Biotechnol J 17:1020-1030, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerman PS Voet D Lawrence MS, et al. : Comprehensive genomic characterization of squamous cell lung cancers. Nature 489:519-525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson JA, Irish JC, Ngan BY: Prevalence of RAS oncogene mutation in head and neck carcinomas. J Otolaryngol 21:321-326, 1992 [PubMed] [Google Scholar]
- 28.Anderson JA Irish JC Mclachlin CM, et al. : H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Arch Otolaryngol Neck Surg 120:755-760, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MS Sougnez C Lichtenstein L, et al. : Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576-582, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen EEW Bell RB Bifulco CB, et al. : The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 7:184, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rampias T Giagini A Siolos S, et al. : RAS/PI3K crosstalk and cetuximab resistance in head and neck squamous cell carcinoma. Clin Cancer Res 20:2933-2946, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Cohen EEW Soulières D Le Tourneau C, et al. : Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393:156-167, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Hanna GJ Guenette JP Chau NG, et al. : Tipifarnib in recurrent, metastatic HRAS-mutant salivary gland cancer. Cancer 126:3972-3981, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho AL Hanna GJ Scholz CR, et al. : Preliminary activity of tipifarnib in tumors of the head & neck, salivary gland, and urothelial tract with HRAS mutations. J Clin Oncol 38, 2016. (suppl; abstr 6504) [Google Scholar]
- 35.Braig F Voigtlaender M Schieferdecker A, et al. : Liquid biopsy monitoring uncovers acquired RAS-mediated resistance to cetuximab in a substantial proportion of patients with head and neck squamous cell carcinoma. Oncotarget 7:42988-42995, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Emburgh BO Arena S Siravegna G, et al. : Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun 7:1-9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gualberto A Scholz C Mishra V, et al. : Abstract CT191: Mechanism of action of the farnesyltransferase inhibitor, tipifarnib, and its clinical applications. Cancer Res 79:CT191-CT191, 2019. (13 suppl) [Google Scholar]
- 38.Strydom N, Rankin SM: Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 5:304-314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niswander LM Fegan KH Kingsley PD, et al. : SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood 124:277-286, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Kura Oncology agrees to share individual participant data that underlie the results reported in this article (after deidentification), including the study Protocol and statistical analysis plan. Data availability will begin 9 months after publication and will be available 36 months after publication. To gain access, data requestors should submit a proposal to medicalaffairs@kuraoncology.com. Proposals will be reviewed by an independent review committee identified for this purpose.