FIG 1.
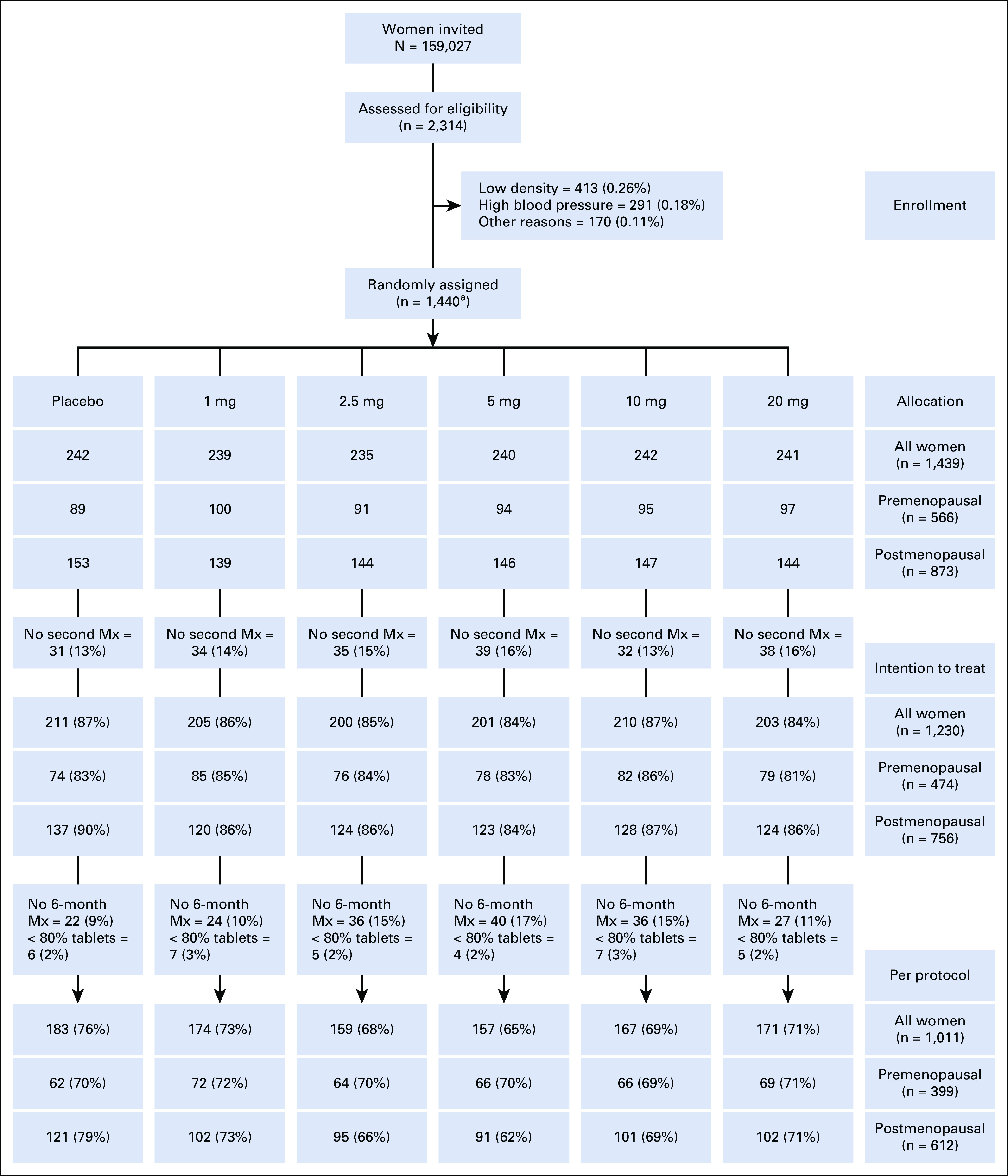
CONSORT diagram for the KARISMA phase II trial. In total, 159,027 women were invited to participate, and 2,314 (1.4%) women were investigated for inclusion to the study. In all, 874 (0.55%) women were excluded because of too low mammographic density (0.26%), hypertension (0.18%), and other reasons (0.11%). The remaining 1,440 (0.9%) women were randomly assigned into placebo, 1, 2.5, 5, 10, or 20 mg of tamoxifen, 240 women in each arm. One randomly assigned participant was found to have no measurable density and was therefore excluded, leaving 1,439 women in the study. A total of 209 participants (14.5%) did not perform a second mammogram and density change could thus not be measured, leaving 1,230 participants in the intention to treat population. Of these women, 185 (12.9%) participants did not complete the full 6-month trial period (but performed an exit mammogram when leaving the trial) or did not take at least 80% of the tablets, 34 (2.4%), leaving 1,011 (70.2%) in the per-protocol population. aOne premenopausal woman was later found to have to measurable density and was excluded, leaving 1,439 in the study. Mx, mammogram.
