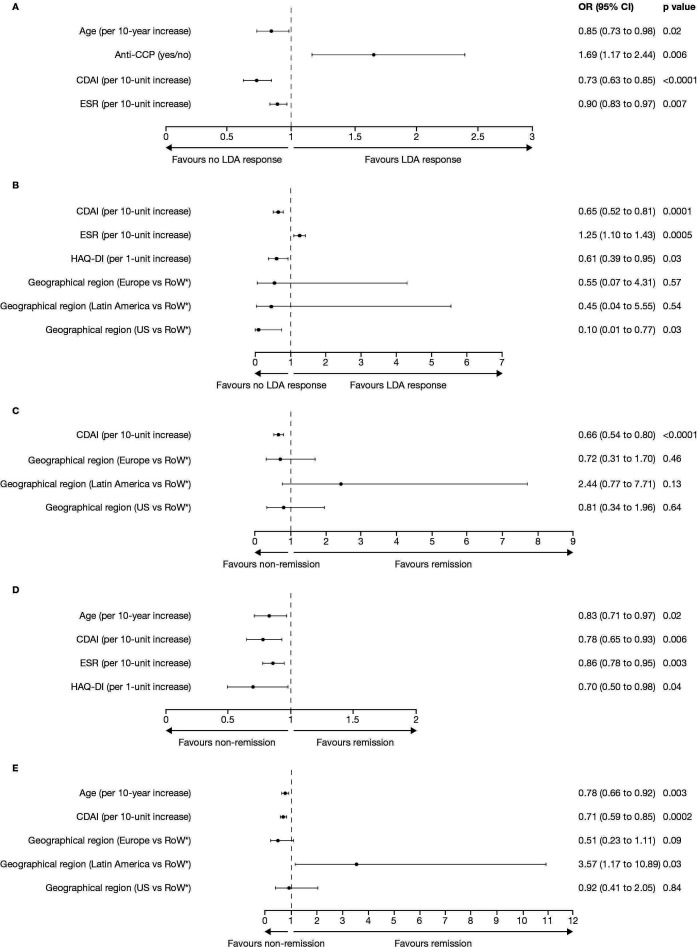
Figure 2.
Multiple logistic regression analyses of baseline predictors of LDA, based on (A) DAS28-4(ESR) ≤3.2 and (B) CDAI ≤10, or remission, based on (C) ACR-EULAR Boolean remission criteria, (D) DAS28-4(ESR) <2.6 and (E) CDAI ≤2.8, with tofacitinib modified-release 11 mg once daily plus methotrexate at week 24 of the open-label phase. *Australia, Philippines, South Korea and South Africa. ACR, American College of Rheumatology; CCP, cyclic citrullinated peptide; CDAI, Clinical Disease Activity Index; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; RoW, Rest of the World.