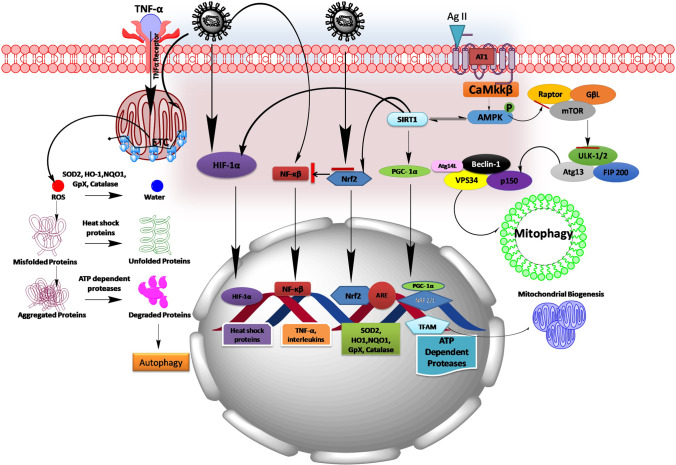
Fig. 3.
Schematic picture showing the plausible mechanism of COVID-19 in mitigating mitochondrial dysfunction. COVID-19 infection stimulates NF-κB pathway and inhibits Nrf2 pathway thereby leads to the redox imbalance and enhances the production of cytokines (TNF-α, IL-1β, IL-6, and IL-10). In turn, TNF-α by acting on its surface receptor enhances the generation of mitochondrial ROS. On another hand, AT1 receptor activation by Ag II formed by ACE involves in regulating AMPK pathway and its downstream mediators controlling mitochondrial function and mitochondrial biogenesis. During the progression of COVID-19 infection, viruses utilize ACE to enter into the host cells and make it unavailable for normal cellular functions, where directly impacts the Ag II homeostasis in controlling cellular functions. This may dysregulate mitochondrial function, perturb mitochondrial biogenesis, and may cause mitochondrial proteotoxicity during COVID-19 infection. Ag II, Angiotensin II; ARE, antioxidant-responsive element; AMPK, adenosine monophosphate–activated protein kinase; Atg, anti-thymocyte globulin; FIP200, FAK family kinase-interacting protein of 200 kDa; GpX, glutathione peroxidase; HIF-1α, hypoxia-inducible factor 1-alpha; HO1, heme oxygenase 1; mTOR, mechanistic target of rapamycin; NQO1, NAD(P)H dehydrogenase [quinone] 1; SIRT1, silent mating–type information regulation 2 homolog 1; NRF, nuclear respiratory factor; Nrf2, nuclear factor erythroid 2 (NFE2)–related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Rheb, Ras homolog enriched in the brain; SOD2, superoxide dismutase 2; TFAM, mitochondrial transcription factor; TSC, tuberous sclerosis proteins; Ulk1, unc-51-like autophagy activating kinase; VPS34, vacuolar protein sorting 34