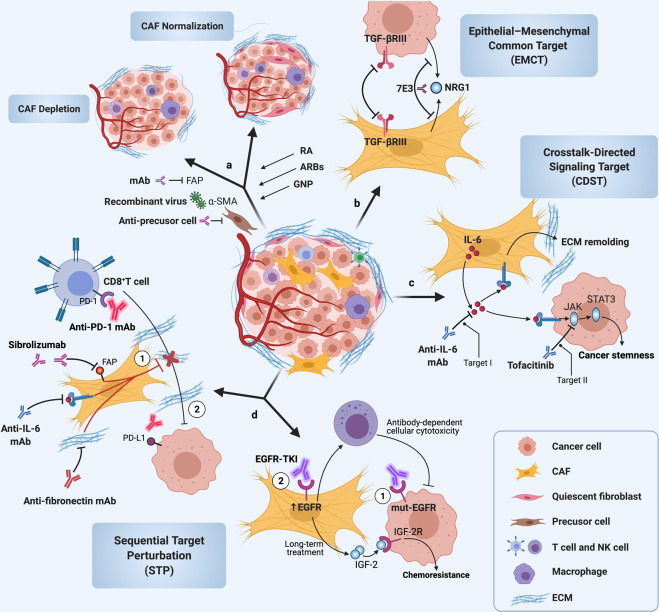
Fig. 7.
CAF-driven targeted therapies and alternative targeting avenues, including epithelial–mesenchymal common target (EMCT), sequential target perturbation (STP), and crosstalk-directed signaling target (CDST) for CAF-directed or host cell-directed antitumor therapy. a Besides the inhibition of NFs to CAFs, there are still two major ways to decrease the CAFs number in TME: (i) targeting specific markers of CAFs by monoclonal antibody (mAb), recombinant virus, anti-precursor cell to deplete CAFs; (ii) revert the activated CAFs to quiescent phenotype by retinoic acid (RA), angiotensin receptor blockers (ARBs), gold nanoparticle (GNP), etc. b Both simultaneous overexpression of the same molecular protein in CAFs and cancer cells like TGF-βRIII and NRG1 has the potential to be targeted as EMCT. c In the regard to CAF-mediated signaling pathways in crosstalk with cancer cells, CDST is targeting simultaneously two different components of one signaling cascade in cancer cells and CAFs, respectively, in which the trigger locating in the upstream of CAFs (Target I), while effector locating in the downstream of cancer cells (Target II). d EGFR-TKI targets the mut-EGFR in cancer cells, while EGFR is up-expressed in CAFs. STP on cancer cells and CAFs, targeting the EGFR in cancer cells firstly (Step①), then in CAFs (Step②), may promote NK cell function to enhance antitumor efficacy and also avoid the IGF-2-mediated chemoresistance after long-term treatment. Since there are different functions in cancer cells and CAFs, STP also aims at targeting CAFs to block the protumor effect firstly (Step①: such as targeting ECM remodeling to move the barrier for CD8+ T cell infiltration) and then aiming at cancer cells (Step②: such as performing PD-1/PD-L1 to antitumor immunotherapy) might obtain well therapeutic efficacy