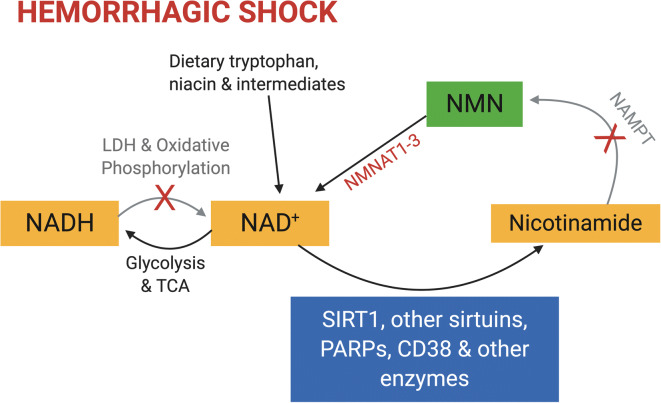
Fig. 3.
NAD salvage pathway and hemorrhagic shock. NAD+ is a key reduction-oxidation (redox) cofactor that accepts high-energy electrons in the form of a hydride ion (H−) during glycolysis and the TCA cycle, generating NADH. Electrons are subsequently donated to complex I of the mitochondrial electron transport chain to support ATP generation, or to pyruvate via lactate dehydrogenase in order to recycle NAD+. NAD also serves as a substrate for several classes of enzymes, including SIRT1 and other sirtuins, which can modulate a broad range of stress responses and other behaviors. These reactions consume NAD, which necessitates regeneration of the pool to support cellular metabolism. Some NAD is synthesized from dietary sources including tryptophan, niacin, and NAD itself or synthetic intermediates, but the bulk of synthesis results from recycling of nicotinamide. This begins with an energetically costly reaction catalyzed by the enzyme NAMPT to make nicotinamide mononucleotide (NMN). NMNAT1–3 then convert NMN to NAD to complete the recycling process. Hemorrhagic shock causes damage and inflammation that can activate NAD consumers such as PARPs and CD38, and may further decrease the levels of NAD by causing a redox shift in favor of NADH due to the limited activity of the electron transport chain in hypoxic conditions, as well as by limiting the NAMPT activity due to lack of ATP. In this case, providing exogenous NMN directly during HS may be especially beneficial by bypassing the NAMPT reaction, thus increasing the NAD levels