Abstract
Background
Recently, some adverse effects of moxibustion has been reported such as burns, smoke, allergies, and so on. To overcome the adverse effects of traditional moxibustion, an ultrasonic moxibustion device (UMD) was designed, simulated, fabricated, and tested. The objective of this study is to provide detailed information about the main design parameters, simulation outcome, and performance-test results.
Methods
The main components of the UMD are a 1-MHz ultrasonic transducer (UT) with concave lens, and its applicator. The acoustic pressure and temperature distribution of the UT was simulated and described graphically using COMSOL software, which is based on the finite element method (FEM). Experimental verification of the temperature distribution was performed on the skin of pork. The temperature-change profiles of pork in relation to increase of therapy time were obtained at an unfocused point (2 mm) and at a focal distance of 13 mm. For the performance test, moxibustion therapy was conducted on the abdominal skin of mice for 120 min using the new UMD and its histological images were acquired to analyze the skin-tissue damage.
Results
The FEM simulation of temperature distribution and acoustic pressure agreed with the experimental outcome. Histological images showed that there was no skin-tissue damage to the mouse abdomens after therapy. The results clearly show that the newly developed UMD can overcome the disadvantages of traditional moxibustion therapy and achieve the proposed design parameters.
Conclusion
The FEM simulation and performance tests provided valuable information about developing future UMDs. In addition, its performance can be compared with traditional moxibustion therapy for future study.
Keywords: Moxibustion, Acupoint, Ultrasonic transducer, FEM simulation
1. Introduction
Moxibustion has recently attracted substantial attention in the Western Pacific region because of a relatively simple and tolerable treatment1,2 and can be applied in treating a variety of diseases such as stroke, pain, malposition, diarrhea, and colitis.3 The use of moxibustion is mainly divided into direct and indirect methods. In direct moxibustion, a cone-shaped portion of moxa (~ 2 mg) is placed on the skin over an acupuncture point and burned.4 Indirect moxibustion is treatment induced by leaving a space between the skin and the moxa.5
The temperature of burning moxa is between ~550 and 890 °C, and Okazaki et al., reported that direct moxibustion was able to raise the temperature to 56 °C inside the skin of a mouse abdomen and to 130 °C on the outside of the skin.4,6 In indirect moxibustion, the maximum temperature was about 65 °C on the mouse skin and 45 °C in the subcutaneous tissue layer.5 However, the safe and effective temperature range for thermal therapy is reportedly from 42 to 44 °C.7,8 The burning moxa can also produce harmful gases and odors9 and the components of moxa smoke are similar to those of tobacco smoke and air pollutants.10 A moxibustion therapy is typically performed for about 20–30 min and patients are often treated several times a week for a few weeks. Ultimately, this exposes patients and acupuncturists to the moxa smoke during therapy.10
To overcome these disadvantages of traditional moxibustion therapy, there have been many studies for developing new technical methods by which to replace conventional moxibustion. These studies have involved options such as near-infrared laser, electric moxibustion, point electrothermal moxibustion, and ultrasonic devices.9,11, 12, 13, 14 However, these studies did not provide detailed information about the process of fabricating UTs, the acoustic fields generated by the ultrasonic moxibustion device (UMD), ultrasonic systems needed to activate the ultrasonic transducers, or the design parameters of the UMD.
For these reasons, the objectives of this study were to provide detailed information about the design parameters, fabrication process, and the performance of the newly developed UMD. The acoustic pressure generated by the UT within a human body was simulated using FEM software (COMSOL Multiphysics, Sweden) and an experiment for acoustic pressure was performed. In addition, temperature profile of developed UT was simulated. For the verification of newly developed UMD, temperature profiles of pork were obtained within the focal distance (FD, 13 mm) and at an unfocused point (2 mm) with increase of time. Finally, moxibustion therapy was performed on mouse-abdomen skin for 120 min. To assess the internal damage, histological images of mouse-abdomen skin were obtained and analyzed after moxibustion therapy.
2. Methods
2.1. Ultrasonic system for the UMD
The detailed description of UT for moxibustion therapy was provided in the Supplementary material. The ultrasonic system was designed to control the power and treatment time of the UMD within the safe temperature range (42 to 44 °C).7,8 Considering the typical moxibustion treatment time (~ 20 to 30 min), 30 min was selected as the treatment time.
The main components of the ultrasonic system were a pulse generator, a power amplifier, a 16-bit microprocessor (dsPIC33F, Microchip Technology, USA), and a miniature thermistor (Murata Electronics, Japan). The tone-burst signal was used to excite the UT due to its high signal-to-noise ratio efficiency.15 The maximum performance of the power amplifier was 50 W with a 50 output port. The input voltage of the ultrasonic system was 48 V. The input parameters of the tone-burst waveform were designed to be controlled by the microprocessor. In addition, because the miniature thermistor was inserted into the UT, the information about real-time temperature change of the UT could be obtained. Fig. 1 shows flow charts of the operating process for the ultrasonic system.
Fig. 1.
Flow charts of the operating process of the ultrasonic system: (a) flow Chart of the tone burst – waveform control, (b) Flow chart of the temperature feedback system. T is the real-time temperature of the specimen.
In Fig. 1a, when generating the tone-burst excitation signals after setting the waveform parameters, the temperature feedback logic is activated simultaneously. In Fig. 1b, T is the real-time temperature of the UT. If the temperature of the UT exceeds the maximum temperature, the UT power is cut off. Otherwise, if the temperature of the skin decreases to less than the minimum temperature, the tone-burst waveform is activated again to excite the UT. This process continues for a maximum 30 min, based on the selected treatment time.
2.2. FEM simulation
The acoustic pressure of fabricated UT in body was simulated by the FEM software and the measured acoustic pressure of the three fabricated UTs was compared with the simulated outcome. In addition, the thermal distribution with increase of the therapy time (up to 300 s) was also performed by FEM simulation to prevent overheating. Thus, the heat sinks were designed and mounted on the external surface of the developed UTs. The length and thickness of the heat sinks (aluminum) were 10 and 2 mm. The thermal distribution includes the piezo-electric element (zirconate titanate, PZT) used, water (ultrasonic couplant), the heat sink, and the designed concave lens.
2.3. Performance test
To verify the performance of the refined UMD, an experiment was performed to obtain the temperature profile of a portion of pork. The operating conditions of the UMD were fixed at 1 MHz, the PRF at 10 Hz, and the pulse duration was varied from 10% to 100%. To analyze the ultrasonic focusing effectivity, the miniature thermistor was inserted into a portion of pork at depths of 2 and 13 mm. Then, the real-time changes in temperature were measured for 1200 s. The experiment was performed at room temperature. This can be possible to read and precisely control the real-time temperature of the pork although it was outside the range of appropriate therapy temperatures. The experimental set-up is shown in Supplement Fig. S1.
Furthermore, the traditional moxibustion therapy was performed using the UMD on the skin of mouse abdomens for 120 minutes. The experimental set up was the same as in Supplement Fig. S1, but laboratory mice were used instead of pork. The traditional moxibustion therapy was performed for 30 min and the UMD was conducted for 120 min to demonstrate its safe and durable performance. To evaluate the damage to mouse skin tissue, histological analysis was performed using two stains (Hematoxylin and eosin: H&E, and Masson's trichrome: MT), which are the most widely used in medical diagnosis. The used reagents were H&E staining kit (ab245880, Abcam, UK) and MT staining Kit (ab150686, Abcam, UK). To perform both stains, the technical methods provided by the staining kit company were used.
3. Results
3.1. FEM analysis
The results of the FEM simulation of the acoustic pressure in the body and their measured acoustic pressure in shown in Fig. 2(a) and (b).
Fig. 2.
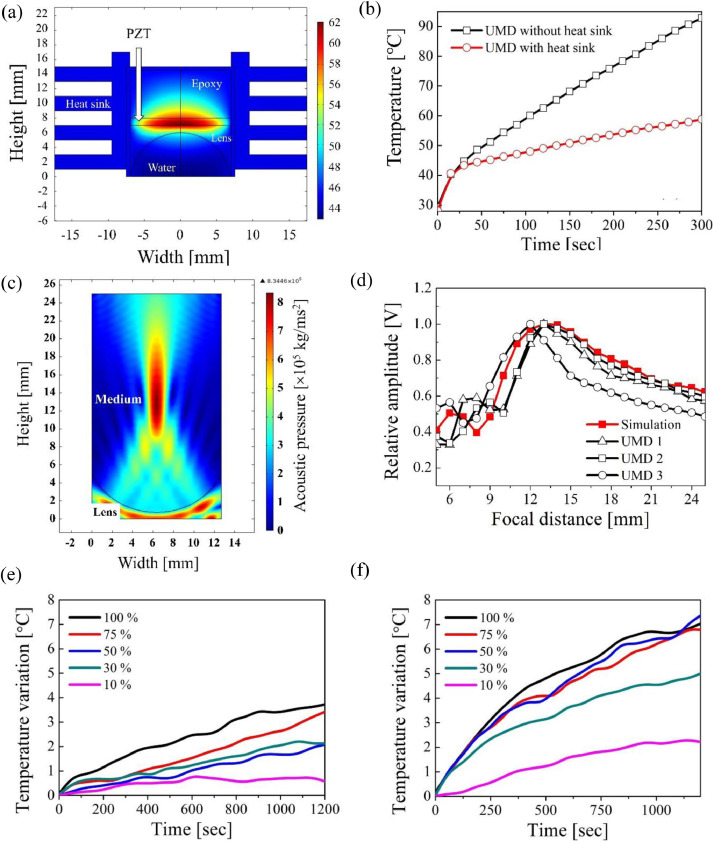
(a) Simulation result of acoustic pressure in the body; (b) Experimental outcome of on axis acoustic pressure of FEM simulation and from the fabricated UMDs; Finite-element-analysis result and the newly developed ultrasonic moxibustion device: (c) Temperature distribution of the ultrasonic transducer with mounted heat sink, in relation to increase in the excitation time; (d) Heating curves of two UTs with and without a heat sink; Temperature profile obtained with increase of the excitation time on pork (10%, 30%, 50%, 75%, and 100% excitation pulse duration): (e) Temperature profile at 2 mm depth and (f) Temperature profile at 13 mm depth.
In Fig. 2 (a), the 1 MHz plane wave was produced from 0 mm and the maximum acoustic pressure (0.83 × 106 kg/ms2) was focused on the FD (13 mm). In Fig. 2 (b), the red line is the simulated on-axis acoustic pressure from the lens center and the black lines are the experimental results from the fabricated UMDs. The measured FD is 13 mm for the first and second UMDs and 12 mm for the third UMD. Fig. 2 (c) and (d) also shows the simulation outcome of the fabricated UT integrated with the heat sinks, and an image of the refined UMD.
In Fig. 2 (c), the center of the PZT produces the highest temperature during its vibration. The temperature curve with increase of excitation time up to 300 s is shown in Fig. 2 (d). The black and red lines are the temperature of the center part with and without the heat sink, respectively. In the beginning, the temperature of both simulations rapidly increases linearly for up to 20 s. The slope of the black lines is almost 2.5 times higher than that of the red line and the maximum temperature of the black line shows an increase up to about 90 °C within 300 s. After adding the heat sink, the temperature increased to about 54 °C.
3.2. Performance test
The result of the performance test of UMD therapy on pork is also shown in Fig. 2 (e) and (f). In Fig. 2 (e), the maximum temperature variation of the 100% pulse duration was about 4 °C at about 900 s. After 900 s, its slope hardly changed (almost zero). The maximum temperature variation of the 75% pulse duration reached 4 °C at about 1200 s and the slopes of the 10%, 30%, and 50% excitation pulse durations were lower than those at 75% and 100%. In Fig. 2 (f), the 50%, 75%, and 100% excitation pulse durations reached 7 °C at about 1000 s and the slopes at 10% and 30% pulse duration barely changed after 1000 s.
Fig. 3 shows the results of the histological analysis according to traditional moxibustion therapy (30 min) and the ultrasonic moxibustion therapy time (0, 30, 60, and 120 min).
Fig. 3.
Hematoxylin and eosin (H&E) and Masson's trichrome (MT)-stained images of mouse abdomen skin after moxibustion therapy: (a) Traditional moxibustion therapy (direct and indirect methods for 30 min) and (b) New ultrasonic moxibustion device (0, 30, 60, and 120 min).
In both images of the direct and indirect method, skin deformation and burning injuries such as epidermis damage, synechia of fibrous tissue, and collagen dissolution on skin tissue occurred when burning moxa. In addition, skin folding, vesiculation, and desquamation were also observed with the indirect method. However, in Fig. 3 (b), the mouse abdomen skin (e.g., collagen in the dermis layer) shows no damage during ultrasonic moxibustion therapy (after 120 min). During the ultrasonic moxibustion therapy, the skin temperature was increased to ~ 6.5 °C after about 16 min and treatment lasted for 120 min.
4. Discussion
4.1. FEM analysis and performance test
In Fig. 2 (a) and (b), the third fabricated UT has a shorter FD than other two UTs because of errors in its machining process. The error range of the machining process was ± 0.5 mm and the measured radius of the concave lens was within the error range. From above results, the proposed design parameters of UT (see Supplement Fig. 2) of UMDs can be used for simulating a focal point of UMDs.
As reported in the aforementioned studies,1,2,9 electric moxibustion devices (EMDs) have been also considered alternatives for the traditional moxibustion method.16 The most different feature between an EMD and a UMD is the focal capacity. In addition, EMD has a much simpler structure and relatively much lower price than that of a UMD. Unlike with EMDs, with UMDs it is possible to restrict the mechanical vibration to a certain point and area. This can accomplish stimulation of an acupoint in a three-dimensional coordinate system.17
4.2. Performance test
From the pork experiment, we found that the acoustic energy could be focused at the FD and also that its temperature increased much faster than at the unfocused point. Also, the ultrasonic focusing ability at the FD for thermal therapy is considered more effective than the unfocused area, and this result is similar to that with the acoustic pressure simulation outcome. Thus, to perform moxibustion therapy, over 50% of the pulse duration was required, and the thermal stimulus point could be controlled using derived design parameters we suggested (see Supplement Fig. S3).
From the performance test on mouse abdomen skin, the temperature was increased to the limit of the safe thermal treatment range and maintained for 120 min without any skin damage. Considering typical moxibustion therapy times (20–30 min), the design parameters and the FEM simulation were effective for designing the UMD and for analyzing its acoustic and thermal characteristics.
In the H&E and MT images, although the treatment time of the ultrasonic moxibustion therapy was much longer than for traditional moxibustion therapy, there was no skin damage. Moreover, temperature variation could be precisely obtained with increase of the time via the UMD. Therefore, developed UMD could effectively perform the moxibustion therapy within safe temperature range without negative side effects.
4.3. Clinical and research implications
The mechanism of moxibustion is mainly related to thermal effects, radiation effects, and pharmacological actions. Among these effects, the thermal-physical effect is the most crucial because moxibustion therapy is basically focused on the application of heat using burning herbal materials.4,18 Because of the heat and burning materials, the burning moxa can cause serious pain and burns, as well as leave scars.19 In addition, unpleasant odors of the burning moxa can occur dizziness, nausea, and throat problems.20 The critical impact on the ultrasonic moxibustion therapy is to overcome these negative effects as well as provide detailed information about the design parameters, fabrication process, and its specification of the UMD.
4.4. Limitations
In general, ultrasonic devices for medical purpose are expensive because high-priced materials and hardware system are required for precise control. Thus, cost-effective UMD should be intensively studied for its popularization. Moreover, it is necessary to study the effective resonance frequency of UMD for absorbing medicinal materials. In this study, although the 1 MHz was used for the sake of the simple design of UMD, the most effective resonance frequency should be defined according to the kinds of the medicinal materials. Therefore, additional studies for developing cost-effective UMD and analyzing the frequency characteristics of various medicinal materials should be conducted.
4.5. Conclusion
As a substitute for traditional moxibustion therapy, a novel UMD was designed, simulated, fabricated, and tested. The new UMD included a 1 MHz UT integrated with a concave lens and an ultrasonic system to control the excitation waveform parameters such as operating frequency, PRF, and pulse duration. The ultrasonic system also includes a real-time temperature feedback system for safe thermal treatment (42 to 44 °C). In terms of the feasibility study to develop the new UMD, based on the simulated and experimental outcomes, we clearly demonstrated a safer and more stable moxibustion therapy than possible using the traditional method, within the temperature range of effective thermal therapy. This opens a promising research avenue to explore.
Funding
This research was supported by National Research Council of Science & Technology (research project G14120).
Ethical statement
This research has been approved by Korea Institute of Oriental Medicine (KIOM).
Data availability
Data will be available upon reasonable request.
CRediT authorship contribution statement
Geonwoo Kim: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Visualization. Young-In Hwang: Software, Formal analysis, Investigation, Resources, Data curation, Visualization. Yeonhee Ryu: Conceptualization, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Hak-Joon Kim: Conceptualization, Methodology, Validation. Young-Min Bae: Conceptualization, Validation, Formal analysis, Investigation, Resources, Data curation. Ki-Bok Kim: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2021.100729.
Appendix. Supplementary materials
References
- 1.Li Y, Sun C, Kuang J. An in vitro and numerical study of moxibustion therapy on biological tissue. IEEE Trans Biomed Eng. 2018;65(4):779–788. doi: 10.1109/TBME.2017.2719633. [DOI] [PubMed] [Google Scholar]
- 2.Park H, Lee I-S, Lee H, Chae Y. Bibliometric analysis of moxibustion research trends over the past 20 years. J Clin Med. 2020;9(5):1254. doi: 10.3390/jcm9051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Q-f, Wu H-g, Liu J, Hong J. Bibliometric analysis of diseases spectrum of moxibustion therapy. J Acupunct Tuina Sci. 2012;10(6):342–348. doi: 10.1007/s11726-012-0633-6. [DOI] [Google Scholar]
- 4.Deng H, Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid-Based Compl Altern Med. 2013:2013. doi: 10.1155/2013/379291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiba A, Nakanishi H, Chichibu S. Effect of indirect moxibustion on mouse skin. Am J Chin Med. 1997;25(02):143–151. doi: 10.1142/S0192415X97000160. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki M, Aizawa S, Yamauchi M, Oguchi K. Effects of single moxibustion on cutaneous blood vessel and microvascular permeability in mice. Am J Chin Med. 1990;18(03n04):121–130. doi: 10.1142/S0192415X90000162. [DOI] [PubMed] [Google Scholar]
- 7.Lepock JR. How do cells respond to their thermal environment? Int J Hyperth. 2005;21(8):681–687. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- 8.Habash RWY, Bansal R, Krewski D, Alhafid HT. Thermal therapy, Part 1: an introduction to thermal therapy. Crit Rev Biomed Eng. 2006;34(6):459–489. doi: 10.1615/critrevbiomedeng.v34.i6.20. [DOI] [PubMed] [Google Scholar]
- 9.Kang HR, Jung CY, Lee SD, Kim KH, Kim KS, Kim EJ. Efficacy and safety of electrical moxibustion for knee osteoarthritis: study protocol for a randomized controlled trial. Trials. 2018;19(1):1–9. doi: 10.1186/s13063-018-2514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Zhao B, Huang Y. Effects of moxa (Folium Artemisiae argyi) smoke exposure on heart rate and heart rate variability in healthy young adults: a randomized, controlled human study. Evid-based Compl Altern Med. 2013:2013. doi: 10.1155/2013/510318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo M, Zhao L, Wu F. CO2 laser moxibustion for knee osteoarthritis: study protocol for a multicenter, double-blind, randomized controlled trial. Chin J Integr Med. 2020;(20057):1–9. doi: 10.1007/s11655-019-2714-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho J, Prasad B, Kim JK. Near-infrared laser irradiation of a multilayer agar-gel tissue phantom to induce thermal effect of traditional moxibustion. J Innov Opt Health Sci. 2018;11(6):1–12. doi: 10.1142/S1793545818500335. [DOI] [Google Scholar]
- 13.Tsuruoka N, Watanabe M, Seki T, Matsunaga T, Haga Y. Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC’10. 2010. Acupoint stimulation device using focused ultrasound; pp. 1258–1261. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Deng H, Shen X. Safety of moxibustion: a systematic review of case reports. Evid-based Compl Altern Med. 2014:2014. doi: 10.1155/2014/783704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaels JE, Lee SJ, Croxford AJ, Wilcox PD. Chirp excitation of ultrasonic guided waves. Ultrasonics. 2013;53(1):265–270. doi: 10.1016/j.ultras.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Kang B, Jung WM, Lee H, Chae Y. Psychophysical and psychophysiological effects of heat stimulation by electric moxibustion. Compl Ther Med. 2019;42(October 2018):400–405. doi: 10.1016/j.ctim.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim G, Seo M-K, Kim Y-I, Kwon S, Kim K-B. Development of phased array ultrasonic system for detecting rail cracks. Sens Actuat A Phys. 2020;311 doi: 10.1016/j.sna.2020.112086. [DOI] [Google Scholar]
- 18.Chiu JH. How does moxibustion possibly work? Evid-based Compl Altern Med. 2013:2013. doi: 10.1155/2013/198584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi SH. Thermal properties of direct and indirect moxibustion. JAMS J Acupunct Meridian Stud. 2009;2(4):273–279. doi: 10.1016/S2005-2901(09)60068-6. [DOI] [PubMed] [Google Scholar]
- 20.Park JE, Lee SS, Lee MS, Choi SM, Ernst E. Adverse events of moxibustion: a systematic review. Compl Ther Med. 2010;18(5):215–223. doi: 10.1016/j.ctim.2010.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon reasonable request.