FIG 3.
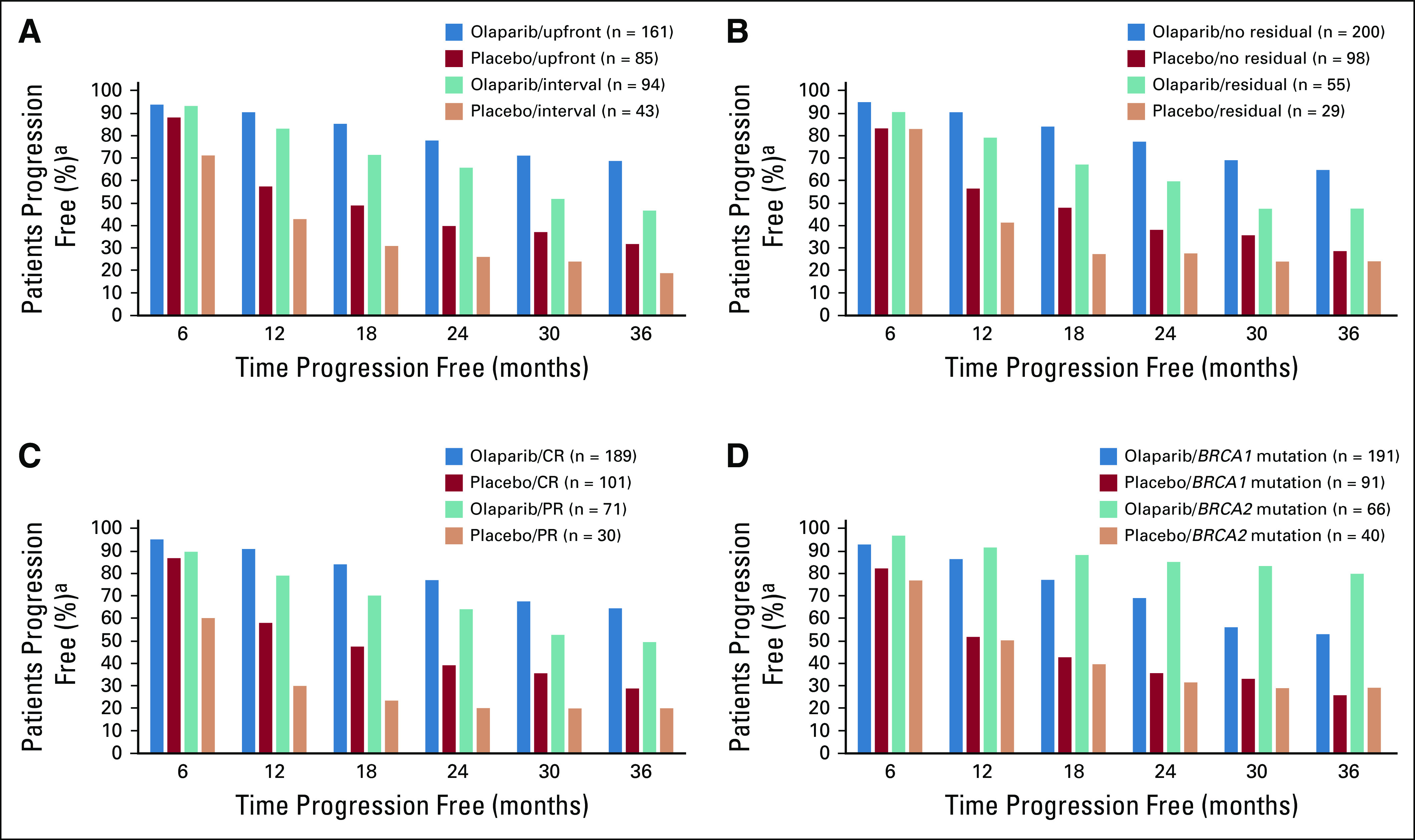
Proportion of patients free of progression or death over time for subgroup analysis–based Kaplan-Meier estimates for (A) surgery timing (8 patients had no surgery or were missing timing data [olaparib arm, n = 5; placebo arm, n = 3]), (B) residual macroscopic disease status, (C) response after platinum-based chemotherapy at baseline, and (D) BRCA mutation status (3 patients [all in olaparib arm] had both BRCA1 and BRCA2 mutations and were progression free up to 42 months). CR, complete response; PR, partial response. (a) Based on Kaplan-Meier estimates.
