Abstract

In this work, the influence of different N2/CO2 contents (up to 60% in fuel volume) on combustion features of laminar-premixed CO/CH4/H2 flame with various equivalence ratios (0.6–1.6) at standard conditions was numerically calculated using ANSYS CHEMKIN-PRO with the GRI-Mech 3.0 mechanism. The mole fraction profiles of the major species and the rate of production of dominant elementary reactions in the flames of CO/CH4/H2/N2/CO2/air were obtained. The effect of inert gas addition on the formation of NOX, H, O, and OH was analyzed, and the sensitivity coefficient of the active radical mole fraction was obtained. The results suggest that the addition of inert gas of the fuel mixture with various equivalence ratios reduces laminar burning velocity and adiabatic temperature, which have always had a good positive correlation and the maximum peak point shifted left. CO2 has obvious inhibitory effect on the formation of NO by reducing the amount of O radicals and obstructing the conduct of the reaction of NNH + O ⇔ NH + NO, but it promotes the formation of NO2 mainly through the reaction HO2 + NO ⇔ NO2 + OH. The reactions H + O2 + H2O ⇔ HO2 + H2O, H + O2 ⇔ O + OH, and OH + CO ⇔ H + CO2 are three very important reactions for the molar fractions of H, O, and OH that decrease significantly with an increase of inert gas concentration.
1. Introduction
With the increasing impact of fossil energy consumption and air pollution on human living environment, it is important to measure energy transformation to promote the development of renewable energy and establish an energy supply system with new and renewable energy as the main body. The energy from biomass (i.e., bioenergy) is considered to be renewable, due to the abundance of perennial bioresources, and carbon-neutral, due to their ability to reabsorb the carbon emitted from combustion of the same.1 The most traditional way to get energy from biomass is to direct fuel combustion.2 However, the biomass plant combustion has very low efficiency and a great amount of pollutant emission. As an alternative, biomass gasification, an energy conversion process including a group of complex chemical reactions wherein large organic molecules degrade into carbon monoxide, methane, hydrogen, and other flammable gases, has been regarded as an effective pathway for utilization of bioresources.3 The components of syngas may change according to the characteristics of biomass, the type of gasifier, the gasification fluid, and the operating parameters.4Table 1 shows the main gas components of different types of biomass gasification processes in the literature.5−14 It can be noticed that the concentration of CO in rice husk gasification is high, and the gas composition ratio of CO/CH4/H2 is close to 2:1:1. In addition, CO2 and N2 account for about 15–65% in most gasification gases. As known, the thermal, chemical, and dilution effects of inert gases will have a certain impact on the combustion characteristics of hydrocarbon fuel. In this paper, the effects of different inert gas rates (0–60%) on the combustion characteristics of CO/CH4/H2 (volCO/volCH4/volH2 = 2:1:1) are numerically studied, which are based on the actual biomass gasification gas composition, for obtaining more data on the effect of inert gas on the combustion characteristics of biomass gas.
Table 1. BDG Composition (Molar Fraction) from Different Biomass Sources and Conversion Processesa.
| syngas
composition (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| BDGs | CO | CH4 | H2 | CO2 | N2 | biomass | reaction agent | type of gasifier | ref |
| GG-W | 20.67 | 11.11 | 5.37 | 15.52 | 47.33 | rice husk | air | CFBG | (5) |
| GG-M | 16.67 | 9.77 | 8.96 | 21.68 | 42.92 | rice husk | air | CFBG | (6) |
| GG-X | 16.9 | 9.77 | 10.01 | 20.13 | 43.19 | rice husk | air | BFBG | (7) |
| GG-K | 6.32 | 13.74 | 14.65 | 5.15 | 53.09 | mixed plastic wastes | air | TSG | (8) |
| GG-M | 19.25 | 0.3 | 17.1 | 11.05 | 52.3 | rubber wood | steam + air | DDG | (9) |
| GG-M | 32 | 0.13 | 50 | 8 | 8.87 | wood chip | steam | MS | (10) |
| GG-R | 13.99 | 4.82 | 19.53 | 18.36 | 43.3 | oil palm trunk | steam + air | DDG | (11) |
| GG-W | 38 | 2.8 | 36 | 21 | 2.3 | cotton stalk pellets | pure O2 | TSG | (12) |
| GG-B | 32 | 33 | 11 | 27 | 5 | almond shells | steam + O2 | BFBG | (13) |
| GG-L | 37.65 | 4.78 | 27.17 | 28.89 | 1.51 | sewage sludge | steam + O2 | DDG | (14) |
CFBG = circulating fluidized bed gasifier, BFBG = bubbling fluidized bed gasifier, TSG = two-stage gasifier, DDG = downdraft fixed-bed gasifier, MS = modeling and simulation.
In recent years, the effects of inert gases (CO2 and N2) on the combustion characteristics of hydrocarbon fuel mixture have been studied by experiments and numerical simulations. Giurcan et al.15investigated the chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames. The numerical results showed that the chemical effect of CO2 is stronger than N2 and mainly occurs through the following reaction: CO + OH ↔ CO2 + H competes with H radicals in reactive H + O2 ↔ O + OH, thus reducing the overall reaction rate and significantly reducing the combustion speed of methane and hydrogen premixed flame. Galmiche et al.16 and Hu et al.17 studied the effects of diluents on the laminar burning velocity of methane/air mixtures. They found that the dilution effect of N2 and CO2 is the main factor to reduce the laminar burning velocity and the thermal-diffusion and chemical effects can be negligible for N2. Burbano et al.,18 Hu et al.,19 and Weng et al.20 investigated the dilution effect of N2/CO2 on the laminar burning velocity and flame stability of H2–CO–O2 mixtures. It was found that, for a certain dilution fraction, the reduction in laminar burning velocity is largely independent of the equivalence ratio and fuel H2–CO mole fraction. Besides, an increase in the N2 and CO2 dilution fractions considerably reduced the laminar burning velocity due to a decrease in heat release and an increase in heat capacity, and this effect was higher for the case of CO2 due to its higher heat capacity and dissociation effects during combustion. Zhang et al.21 experimentally determined that the flame temperature profile and flame computation were conducive to analyze the effects of carbon dioxide and nitrogen dilution on methane/air flames. Results indicated that carbon dioxide addition has more significant effects on the thermal properties of flame, except for flame thickness. Mitu et al.22 studied the influence of inert gas (He, Ar, N2, or CO2) on the laminar burning velocity of methane–air mixtures. They found that dilution with increasing amounts of additives determines the decrease of laminar burning velocity and maximum flame temperature among all investigated compositions of the methane–air mixtures.
Although the flames of pure CH4 and CO/H2 syngas diluted by nitrogen and carbon dioxide have been extensively studied and the effects of nitrogen and carbon dioxide dilution on the combustion are well explored, the literature on the effects of inert gases on combustion characteristics of biomass gases is relatively scarce and the inhibition effect of inert gases on flame combustion is different. Under different inert gas concentrations, the ignition limit, flame stability, combustion heat value, and other parameters of the new tertiary CO/CH4/H2 mixture are not clear enough. Moreover, there is need for obtaining detailed parameters for boiler combustion and internal combustion engine operation in a biomass gasification power plant. Prevention of explosion of this new type of tertiary fuel must be considered during transportation, fuel usage, and storage. Therefore, it is quite necessary to understand the effect of inert gases on the adiabatic temperature and laminar combustion velocity of fuels.
This work aims to obtain the effects of different CO2/N2 contents (up to 60% in fuel volume) on combustion features of laminar premixed biosyngas flame using ANSYS CHENKIN-PRO. The laminar burning velocities and adiabatic temperatures of H2/CO/CH4/CO2/N2/air mixtures have been numerically calculated with various equivalence ratios (0.6–1.6). The thermal-diffusion and chemical effects on the flame structure, including element species mole fraction, production rate, and net reaction speeds of main reactions, are quantitatively discussed. The mole fraction and formation mechanism of NOX and O, H, and OH active radicals under different CO2/N2 contents are analyzed. Besides, the sensitivity coefficient of the main reaction to the molar fraction of the active radicals is discussed.
2. Mechanism and Model
2.1. Mechanism Validation and Computational Methods
In the research, the laminar burning velocities and adiabatic temperatures of CO/CH4/H2/CO2/N2/air mixtures were numerically simulated in CHEMKIN-PRO. A steady, adiabatic, one-dimensional freely propagating flame model was used to determine premixed laminar flame speeds by the PREMIX code.23 The models of premixed flame can solve a set of governing differential equations, such as the steady-state mass, species, and energy conservation equations. Meanwhile, the Soret effect and multicomponent transport model were taken into consideration. In the calculation process, the computational domain was set from −2 to 10 cm and GRAD and CURV values were set at 0.1 to ensure that the maximum number of grids was 900, which satisfied the calculation requirement. Furthermore, this number proved sufficient in rendering the simulation as grid-independent.
One-dimensional flow with uniform inlet conditions was assumed. The governing conservation equations were reduced to the following:
Continuity
| 1 |
Energy
| 2 |
Species
| 3 |
Equation of state
| 4 |
where x represents the one-dimensional coordinates, Ṁ represents the total mass flow rate of gas components, Yk denotes the kth species mass fraction, T represents the temperature in the x direction, P represents the pressure in the x direction, ρ means the density of the mixture, u represents the velocity of the mixed fluid in the x direction, Wk represents molecular weight of the kth species, R is the general constant of gas, W̅ represents the average molecular weight of the mixture, λ is the heat conductivity of the gas, cp represents the heat capacity of the mixed gas at constant pressure, cpk expresses the kth species heat capacity at constant pressure, ω̇k is the production molar rate through the chemical reaction of the kth species every unit volume, Vk indicates the diffusion rate of the kth gas, hk indicates the specific enthalpy of the kth gas, Q̇rad expresses the radiation heat loss of mixture, and A indicates the cross-sectional area of the flow tube.
The flame speed calculator simulates a freely propagating flame at 298 K and 1 atm, the fixed-flame coordinate system was established, and the initial flow rate parameters of flue/air mixtures were set between 0.01 and 0.08 g/(cm2·s). Table 2 shows the reactants’ mole fractions for the computational flames. The inert gas ratio is defined as Xinert = (nCO2 + nN2) × 100%/(nCO + nH2 + nCH4 + nCO2 + nN2), where the mole fractions of Xinert is 0, 30 and 60% in the present calculation and consistent with the proportion of inert gas in the actual gasification gas component. In addition, to make the calculation more accurate, the flue/air mixture flow rate in “C1-Inlet” of the model decreases with an increase of the proportion of inert gas.
Table 2. Reactants Mole Fractions of the Calculated Flame.
| gas
composition (vol %) |
||||||
|---|---|---|---|---|---|---|
| flame no. | CO | CH4 | H2 | CO2 | N2 | inert gas ratio (%) |
| 1 | 50 | 25 | 25 | 0 | 0 | 0 |
| 2 | 35 | 17.5 | 17.5 | 0 | 30 | |
| 3 | 35 | 17.5 | 17.5 | 10 | 20 | |
| 4 | 35 | 17.5 | 17.5 | 20 | 10 | 30 |
| 5 | 35 | 17.5 | 17.5 | 30 | 0 | |
| 6 | 20 | 10 | 10 | 0 | 60 | |
| 7 | 20 | 10 | 10 | 20 | 40 | 60 |
| 8 | 20 | 10 | 10 | 40 | 20 | |
| 9 | 20 | 10 | 10 | 60 | 0 | |
In the present work, GRI-Mech was employed as the chemical mechanism to simulate the premixed laminar flame speed in CHEMKIN-PRO. The GRI 3.0 mechanism consists of 325 elementary chemical reactions with associated rate coefficient expressions and thermochemical parameters for the 53 species.24 It has been widely used in the numerical simulation of hydrocarbon fuel combustion and has been validated by a great amount of experimental data. Figure 1 shows the adiabatic temperature and laminar burning velocities for F1 and methane combustion in three widely acknowledged reaction mechanisms: GRI-Mech 3.0,25 USC-Mech,26 and San Diego-Mech.27 It can be seen from Figure 1 that the results of adiabatic temperature and laminar burning velocities calculated with these mechanisms are in well agreement. Furthermore, the computed methane laminar combustion velocity is also in well agreement with the experimental data from some literature works.22,28−32 Moreover, the optimized GRI-Mech 3.0 mechanism includes the NOx formation reaction and is hence selected.
Figure 1.
(a) Adiabatic temperature and (b) laminar flame speed of H2/CO/CH4/air mixtures and pure methane at various equivalence ratios.
2.2. Sensitivity Analysis
CHEMKIN-PRO sensitivity analysis is used to solve the results of flame experiments. Sensitivity analysis not only gives researchers a deep understanding of the main control parameters of the reaction process but also can be applied for uncertainty analysis, that is, characterizing the uncertainty of model output as a result of improperly known parameters.33
In some times, the sensitivity coefficients are often normalized expressed with eq 5
| 5 |
where SFi denotes the sensitivity coefficient, αi means the constants of the reaction rate, and Xkmax means the maximum species mole fraction.
2.3. Adiabatic Temperature
The adiabatic temperature was computed by gas-phase equilibrium program EQUIL.34 The gas-phase chemistry (chemical reactions and rate parameters) and thermochemical data required for both are also based on GRI 3.0. In numerical simulations, the unburnt gas mixture has been assumed to be in a closed adiabatic system under constant pressure conditions.
3. Results and Discussion
3.1. Adiabatic Temperature and Laminar Burning Velocity
Figure 2 shows the variation distribution of the adiabatic temperature and laminar burning velocity of the mixture of different H2/CO/CH4/CO2/N2 components (as shown in Table 1) simulated by CHEMKIN. It shows that the adiabatic temperature and laminar burning velocity increasing first and then decreasing like the expected results have a good positive correlation yet at different equivalence ratios under atmosphere conditions (298 K and 1 atm), which have a negative correlation with the increasing concentration of inert gases. Furthermore, the maximum peak point shift significantly to the left with an increment of inert gas concentration. With the proportion of inert gas increasing from 0 to 60%, the equivalence ratio corresponding to the peak adiabatic temperature decreases from 1.05 to 1 and the temperature decreases by about 478 K. Meanwhile, the laminar burning velocity is also decreased significantly and the equivalence ratio corresponding to its maximum peak value decreases from 1.25 to 1, which is decreased by about 62 cm/s.
Figure 2.
(a) Computed adiabatic temperature and (b) laminar combustion velocity of different fuel components.
The influence of inert additives on laminar burning velocity was assigned mostly to their ability to change the thermal properties of flammable mixtures by changing the heat capacity and consequently the flame temperature.22 The simulation results of this work are similar to those reported for pure methane earlier.35 The increase in the concentration of inert gases dilutes the concentration of fuel, leading to a decrease in heat release and reaction rate. On the other hand, it also influences the thermal-diffusion coefficient and oxidation reaction kinetics of the mixture.36,37 As shown in Figure 2, the ability of CO2 to affect the flame propagation speed of fuel is obviously stronger than that of N2. According to the literature, CO2 has a stronger chemical effect and heat capacity than N2, and it has also been found that CO2 is directly involved in chemical reactions through CO + OH ↔ H + CO2.35,38,39
3.2. Flame Structures
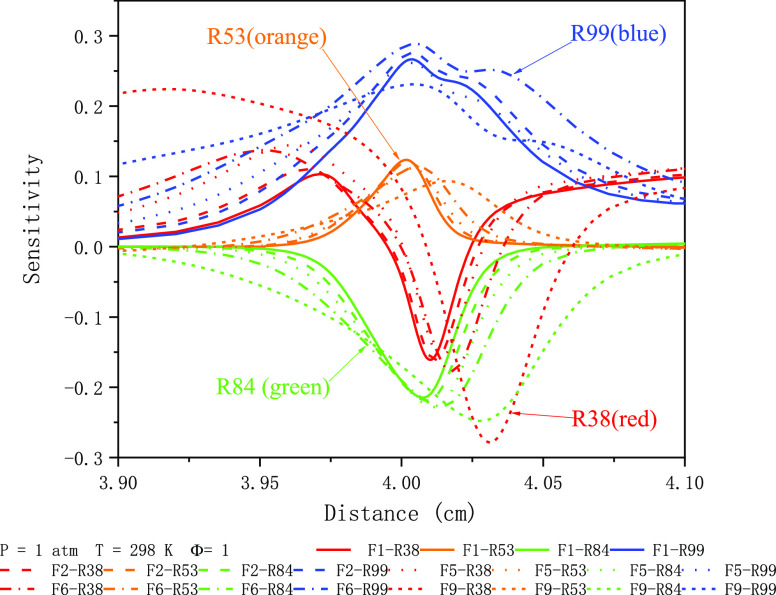
Figure 3 shows the flame structure profiles and production rates of the laminar-premixed flame with different components of the fuel mixture (F1, F2, F5, F6, and F9) at the equivalence ratio Φ = 1, P = 1 atm, and Tu = 298 K. As can be seen, the combustion reaction occurs mainly in the range of 3.92–4.02 cm. Moreover, the reaction of H2 always takes precedence over CO and CH4. ALso, this priority becomes more pronounced as the concentration of CO2 increases. The four main element reactions of H2 combustion are H + O2 ⇔ O + OH (R38), H + CH4 ⇔ CH3 + H2 (R53), OH + H2 ⇔ H + H2O (R84), and OH + CO ⇔ H + CO2 (R99). The sensitivities of H2 reactions at different inert gas concentrations are shown in Figure 4. The results show that R53 and R99 mainly promote the generation of H2, while R38 and R84 mainly promote the consumption of H2. When the CO2 concentration increases from 30 to 60%, the sensitivity coefficient of R38 decreases obviously, which promotes the consumption of H2. This may be due to the reaction of CO2 and hydrogen with reverse water gas, which consumes a certain amount of hydrogen.
Figure 3.

Flame structure profiles and production rates of fuel mixtures with different compositions: (a, d) F1, CO2 = 0 N2 = 0; (b, e) F2, CO2 = 0 N2 = 30%; F6, CO2 = 0 N2 = 60%; and (c, f) F5, CO2 = 30% N2 = 0; F9, CO2 = 60% N2 = 0.
Figure 4.
Sensitivity of the four main element reactions of H2 at different inert gas concentrations.
In addition, the decrease in the molar fraction of CO is always slower than that of H2 and CH4 and may also be related to the early positive production rate. With an increase of inert gas concentration, the sensitivity of the element reaction R99, OH + CO ⇔ H + CO2, which mainly affects the concentration of CO, becomes larger, thus reducing the consumption of CO concentration.
This observation is consistent with the results of the effect of CO production rate on molar fraction proposed by Zhou et al.2
CH4 consumption occurs mainly through the following reactions: H + CH4 ⇔ CH3 + H2 (R53), OH + CH4 ⇔ CH3 + H2O (R98), O + CH4 ⇔ OH + CH3 (R11), OH + CO ⇔ H + CO2 (R99), and H + O2 ⇔ O + OH (R38). When inert gas is not included, R53 plays a dominant role. However, with an increase of inert gas concentration, the sensitivity coefficient of R99 decreases obviously, and the consumption of CH4 is much far more than that of R53.
Figure 5 presents the net reaction rates of ten main elemental reactions that were selected at different inert gas concentrations during combustion. Among them, R38 (H + O2 ⇔ O + OH) is an important chain branching reaction in the combustion process, producing a larger number of O and OH active radicals, which is of great significance to the adiabatic temperature of fuel combustion and the velocity of laminar flame propagation. As shown in Figure 5, with the addition of inert gases, the net reaction rate of each reaction is reduced by an order of magnitude. In addition, with the concentration of CO2 from 30 to 60%, the maximum peak of the net reaction rate of all reactions is shifted to the right, while the reaction interval is elongated, thus reducing the laminar flame speed of the fuel mixture.
Figure 5.

Net reaction rates of main reactions at different inert gas concentrations: (a) F1, CO2 = 0 N2 = 0; (b) F2, CO2 = 0 N2 = 30%; (c) F6, CO2 = 0 N2 = 60%; (d) F5, CO2 = 30% N2 = 0; and (e) F9, CO2 = 60% N2 = 0.
3.3. Effect of Inert Gas Addition on NOX Formation
NOX have a great influence on air pollution. NOX is formed, on the one hand, by the nitrogen element in the fuel and, on the other hand, mainly by the oxidation of N2 in the air. The effects of inert gas on NOx production from CH4 combustion have been reported in previous literature works.40,41Figure 6 shows the effects of different inert gas concentrations on the formation of NO, N2O, and NO2. As shown in the diagram, CO2 has the obvious inhibitory effect on the formation of NO but it is a promoting effect on NO2 during in the combustion process. However, when the gas concentration reaches equilibrium in the whole reaction process, the molar fraction of NOX is positively correlated with the nitrogen content in the fuel component at the same concentration of the inert gas. In addition, since the molar fraction of NO is much greater than the order of magnitude of N2O and NO2, the amount of NOX in the whole reaction decreases with increasing N2 and CO2 concentrations. The results of this paper are similar to those reported by Xiang et al.42 on the effect of CO2 on the combustion of methane and NOx formation.
Figure 6.

Effects of different concentrations of inert gases on NOx formation: (a) NO, (b) NO2, and (c) NOx.
Figure 7 shows the production rate of the main NO reaction at different inert gas concentrations. The formation of NO is mainly through the following reaction: NNH + O ⇔ NH + NO (R208), N + OH ⇔ NO + H (R180), HNO + H ⇔ H2 + NO (R214), N + O2 ⇔ NO + O (R179), and NH + O ⇔ NO + H (R190). In general, the extended Zeldovich mechanism in the high-temperature region contains a chain reaction R178, R179, and R180 that dominates the formation of NO. However, in this paper, the element reaction R208 has a higher rate of production of NO in all working conditions. The NNH mechanism was proposed by Bozzelli et al.43 and verified by Harrington et al.44 and Hayhurst et al.45 At high temperatures, when the hydrogen atom is more than 0.1%, NNH is formed rapidly by the reaction of H + N2 ⇔ NNH. When the concentration of the inert gas is small, the reaction of NNH + O ⇔ NH + NO (R208) is very important for the amount of NO produced by the fuel mixture in air, as shown in Figure 5. However, with an increase of inert gas concentration, especially the concentration of CO2 from 30 to 60%, the reaction temperature and the number of H/O/OH active radicals decreased, resulting in a significant decrease in the formation rate of all reactions. Thus, the amount of NO is reduced. As shown in Figure 6, in the combustion process, as the concentration of the inert gas increases, the amount of NO2 produced increases as opposed to the result of NO. This is due to the formation of NO2 mainly through reaction HO2 + NO ⇔ NO2 + OH (R186) and consumption through reaction NO2 + H ⇔ NO + OH (R189). Meanwhile, the formation of HO2 free radicals is mainly through reaction H + O2 + M ⇔ HO2 + M (R33) at low temperatures.
Figure 7.
Production rate of NO under different inert gas components: (a) F1 CO2 = 0 N2 = 0; (b) F2 CO2 = 0 N2 = 30%; F6 CO2 = 0 N2 = 60%; and (c) F5 CO2=30% N2 = 0; F9 CO2 = 60% N2 = 0.
3.4. Effect of Inert Gas Addition on the Formation of H, O, and OH Active Radicals
H, O, and OH active radicals play an essential role in combustion reactions.13,46−49 They exist in almost every elementary reaction including chain initial reaction, chain propagation reaction, and chain termination reaction. The reaction rate of the whole system can be directly affected by the process of impact, decomposition, reduction, and oxidation. Therefore, the effect of different inert gas concentrations on the formation of active radicals in the combustion process of CO/CH4/H2/air mixture is also analyzed in this paper. Figure 8 shows the molar fraction distribution of H, O, and OH active radicals at different inert gas concentrations. At the same time, to understand the effect of CO2/N2 on the main elements of active radicals, the sensitivity analysis of H, O, and OH active radicals under different inert gas concentrations is presented in Figure 9. As shown in Figure 8, the molar fractions of H, O, and OH decrease significantly with an increase of inert gas concentration, consistent with the expected results. Moreover, the inhibition of CO2 is significantly stronger than that of N2. The content of CO2 in pure CO/CH4/H2/air mixture increases to 30% and 60%, the maximum molar fractions of H, O and OH active radicals are almost reduced by nearly 40% and 80%, respectively. When the content of N2 increases to 30% and 60%, the maximum molar fractions of H, O and OH active radicals are almost reduced by nearly 20% and 50%, respectively.
Figure 8.
Mole fraction distributions of active radicals under different inert gas components: (a) H, (b) O, and (c) OH.
Figure 9.

Sensitivity analysis of H, O, and OH active radicals under different inert gas concentrations: (a) H, (b) O, and (c) OH.
The normalized logarithmic sensitivity coefficient of the H and O mole fractions with respect to the reaction rate coefficients of each elementary reaction is very similar, but it is different from that of OH, as shown in Figure 9. The chain branching/propagating reactions, H + O2 ⇔ O + OH (R38), OH + CH3 ⇔ CH2(S) + H2O (R97), OH + CO ⇔ H + CO2 (R99), HCO + M ⇔ H + CO + M (R167), O + CH3 ⇔ H + H2 + CO (R284), show positive sensitivity coefficients, which contribute to promote the formation of free radicals. At the same time, it can be seen from the diagram that the content of inert gas has a great effect on elemental reaction R38. When the CO2 content increases to 60%, R38 has the largest positive sensitivity coefficient and far exceeds the other flames. However, R99 always has a high positive sensitivity coefficient and is less affected by inert gas. The results indicate that R38 and R99 play an important role in the production of active free radicals and the increase of reaction rate. On the contrary, the chain termination reactions, H + O2 + M ⇔ HO2 + M (R33), H + O2 + H2O ⇔ HO2 + H2O (R35), H + O2 + N2 ⇔ HO2 + N2 (R36), H + OH + M ⇔ H2O + M (R43), H + CH3(+M) ⇔ CH4(+M) (R52), are constantly consuming active free radicals, diminishing the concentration of free radicals, impeding the reaction. Among them, R35 has the most negative sensitivity coefficient and generates a larger number of low-active HO2 radicals and combustion products (H2O), which affect the collision efficiency of the third body recombination and inhibit the reaction. With an increase of inert gas content, this phenomenon also becomes more obvious. In addition, it can be seen from the sensitivity coefficient of OH that when the N2 content is up to 60%, the chain termination reactions, H + O2 + H2O ⇔ HO2 + H2O (R35), H + O2 + N2 ⇔ HO2 + N2 (R36), H + OH + M ⇔ H2O + M (R43), H + CH3(+M) ⇔ CH4(+M) (R52), 2OH(+M) ⇔ H2O2(+M) (R85), have a higher negative sensitivity coefficient. With an increase of N2 content, the ability of R36 and R43 to reduce free radicals was stronger than that of CO2. This may be due to the chemical effects of direct participation of N2 in the reaction.50−52
3.4.1. H
Figure 10 shows the production rate of H active radicals under different inert gas components. As can be seen from Figure 10, the formation and consumption of H are mainly through the following reactions: OH + H2 ⇔ H + H2O (R84), OH + CO ⇔ H + CO2 (R99), O + H2 ⇔ H + OH (R3), O + CH3 ⇔ H + CH2O (R10), O + CH3 ⇔ H + H2 + CO (R284), H + O2 ⇔ O + OH (R38), H + CH4 ⇔ CH3 + H2 (R53), H + CH2O ⇔ HCO + H2 (R58), H + CH3(+M) ⇔ CH4(+M) (R52), and H + HO2 ⇔ 2OH (R46). Among them, R84, R99, R3, R10, and R284 have positive rates of production to promote the formation of H radical, while the others are negatively consumed radicals. R84 and R38 have the most positive rate of production and the most negative rate of production, respectively. Also, the production rate of each reaction also decreases with an increase of inert gas concentration in all reactions.
Figure 10.
Production rate of H active radicals under different inert gas components. (a) F1 CO2 = 0 N2 = 0; (b) F2 CO2 = 0 N2 = 30%; F6 CO2 = 0 N2 = 60%; and (c) F5 CO2 = 30% N2 = 0; F9 CO2 = 60% N2 = 0.
3.4.2. O
Figure 11 shows the production rate of O active radicals under different inert gas components. The formation and consumption of O are mainly through the following reactions: H + O2 ⇔ O + OH (R38), O + CH3 ⇔ H + CH2O (R10), O + H2 ⇔ H + OH (R3), O + CH4 ⇔ OH + CH3 (R11), O + CH3 ⇔ H + H2 + CO (R284), O + CH2O ⇔ OH + HCO (R15), and 2OH ⇔ O + H2O (R86). R38, which has a positive rate of production, is the most important element reaction for the formation of O radicals. The other reactions have a negative production rate, among which R3 has the most negative production rate and it mainly consumes O radicals. Meanwhile, it can be seen from the diagram that the reaction of elements can convert them into H and OH radicals by consuming O radicals, which act as an intermediate. Moreover, all production rates and consumption rates decrease with an increase of inert gas content.
Figure 11.
Production rate of O active radicals under different inert gas components: (a) F1 CO2 = 0 N2 = 0; (b) F2 CO2 = 0 N2 = 30%; F6 CO2 = 0 N2 = 60%; and (c) F5 CO2 = 30% N2 = 0; F9 CO2 = 60% N2 = 0.
3.4.3. OH
Figure 12 shows the production rate of OH active radicals under different inert gas components. The formation and consumption of OH are mainly through the following reactions: H + O2 ⇔ O + OH (R38), H + HO2 ⇔ 2OH (R46), O + H2 ⇔ H + OH (R3), 2OH ⇔ O + H2O (R86), O + CH4 ⇔ OH + CH3 (R11), OH + H2 ⇔ H + H2O (R84), OH + CO ⇔ H + CO2 (R99), OH + CH4 ⇔ CH3 + H2O (R98), OH + CH2O ⇔ HCO + H2O (R101), and OH + CH3 ⇔ CH2(S) + H2O (R97). As shown in Figure 12, R38 and R46 mainly generate OH by consuming H radicals, while R3, R86, and R11 mainly generate OH by consuming O radicals. All of these reactions contribute to the formation of OH. Consumption reactions are mainly through R84 and R99, which are also the main sources of H radicals. In addition, as shown in Figure 12a, when there is no inert gas, R99 has the most negative formation rate, which is main reaction of OH. With the addition of the inert gas, R84 takes precedence over R99. As the concentration of the inert gas increases, the production and consumption of the most element reaction begin to approach zero. Thus, the number of active radicals in the reaction process was reduced and the reaction was inhibited.
Figure 12.
Production rate of OH active radicals under different inert gas components: (a) F1 CO2 = 0 N2 = 0; (b) F2 CO2 = 0 N2 = 30%; F6 CO2 = 0 N2 = 60%; and (c) F5 CO2 = 30% N2 = 0; F9 CO2 = 60% N2 = 0.
4. Conclusions
The influence of different N2/CO2 contents (up to 60% in fuel volume) on combustion features of laminar premixed biosyngas blaze with various equivalence ratios (0.6–1.6) at standard conditions was numerically studied by ANSYS CHEMKIN-PRO with the GRI-Mech 3.0 mechanism. The thermal-diffusion and chemical effects on the flame structure, including element species mole fraction,production rate and net reaction speeds, were quantitatively discussed. The mole fraction and generation mechanism of NOX and H, O, and OH active radicals under different CO2/N2 contents were studied. The primary results are summarized as follows:
-
(1)
In the case of various equivalence ratios, the laminar flame speed has always had a good positive correlation with adiabatic temperature and the maximum peak point shows a left shift under different CO2/N2 contents. The inhibition effect of CO2 is stronger than that of N2, which may be related to the chemical effect and heat capacity of CO2.
-
(2)
In flame structures, the reaction of H2 always takes precedence over CH4 and CO, which becomes more pronounced as the concentration of CO2 increases. The main reactions of H2 consumption are R38 and R84, and the negative sensitivity coefficient increases with an increase of inert gas concentration. Nevertheless, the molar fraction of CO always changes more slowly than those of H2 and CH4. With the addition of inert gases, the net reaction rate of each reaction decreases by an order of magnitude and the maximum peak is shifted to the right, while the reaction interval is elongated, thus reducing the laminar flame speed of the fuel mixture.
-
(3)
CO2 has the obvious inhibitory effect on the formation of NO by reducing the amount of O radicals and obstructing the conduct of the reaction NNH + O ⇔ NH + NO but promotes the formation of NO2 mainly through reaction HO2 + NO ⇔ NO2 + OH.
-
(4)
The molar fractions of H, O, and OH decrease significantly with an increase of inert gas concentration. Moreover, the inhibition of CO2 was significantly stronger than that of N2. The normalized logarithmic sensitivity coefficients of the H and O mole fractions with respect to the reaction rate coefficients of each elementary reaction are very similar, but it is different from that of OH. R35, R38, and R99 are three very important reactions for the molar fractions of OH, O, and H.
Acknowledgments
The authors appreciate the support from the Shandong Province Key Research and Development Program (Major Technological Innovation Project), Large Tractor Hydraulic CVT Intelligent Continuously Variable Transmission Integration Research and Application (2020CXGC010806), the Shandong Province Key Research and Development Program (Major Technological Innovation Project), Construction Machinery Integration Research and Application of Key Technologies for Intelligent Integration and Matching of Vehicle Assembly (2020CXGC011005), the Jinan City Science and Technology Bureau University Institute Innovation Team Project, the Research on Key Technologies of Intelligent Electro-hydraulic Control System for Large Power Platform, and the National Natural Science Foundation of China (No. 51606194).
The authors declare no competing financial interest.
References
- Bhattacharya A.; Datta A.. Laminar Burning Velocity of Biomass-Derived Fuels and its Significance in Combustion Devices. In Sustainable Energy Technology and Policies; Springer, 2018; pp 359–378. [Google Scholar]
- Zhou Q.; Cheung C. S.; Leung C. W.; Li X.; Li X.; Huang Z. Effects of fuel composition and initial pressure on laminar flame speed of H2/CO/CH4 bio-syngas. Fuel 2019, 238, 149–158. 10.1016/j.fuel.2018.10.106. [DOI] [Google Scholar]
- Luo X.; Wu T.; Shi K.; Song M.; Rao Y.. Biomass Gasification: An Overview of Technological Barriers and Socio-Environmental Impact. In Gasification for Low-Grade Feedstock; IntechOpen, 2018; Chapter 1. [Google Scholar]
- Bridgwater A. V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1995, 74, 631–653. 10.1016/0016-2361(95)00001-L. [DOI] [Google Scholar]
- Wu C. Z.; Yin X. L.; Ma L. L.; Zhou Z. Q.; Chen H. P. Operational characteristics of a 1.2-MW biomass gasification and power generation plant. Biotechnol. Adv. 2009, 27, 588–592. 10.1016/j.biotechadv.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Miao Q.; Zhu J.; Barghi S.; Wu C. Z.; Yin X. L.; Zhou Z. Q. Modeling biomass gasification in circulating fluidized beds. Int. J. Energy Power 2013, 2, 57–63. [Google Scholar]
- Xiong Q.; Yeganeh M. M.; Yaghoubi E.; Asadi A.; Doranehgard M. H.; Hong K. Parametric investigation on biomass gasification in a fluidized bed gasifier and conceptual design of gasifier. Chem. Eng. Process. Process Intensif. 2018, 127, 271–291. 10.1016/j.cep.2018.04.003. [DOI] [Google Scholar]
- Kim J. W.; Mun T. Y.; Kim J.; Kim J. S. Air gasification of mixed plastic wastes using a two-stage gasifier for the production of producer gas with low tar and a high caloric value. Fuel 2011, 90, 2266–2272. 10.1016/j.fuel.2011.02.021. [DOI] [Google Scholar]
- Giurcan V.; Mitu M.; Razus D.; Oancea D. Laminar Flame Propagation in Rich Ethane-Air-Inert Mixtures. Rev. Chim. 2016, 67, 1084–1089. [Google Scholar]
- Mehrpooya M.; Khalili M.; Sharifzadeh M. M. M. Model development and energy and exergy analysis of the biomass gasification process (Based on the various biomass sources). Renewable Sustainable Energy Rev. 2018, 91, 869–887. 10.1016/j.rser.2018.04.076. [DOI] [Google Scholar]
- Giurcan V.; Mitu M.; Movileanu C.; Razus D. Temperature, pressure and dilution effect on laminar burning velocity of propane-air. Rev. Roum. Chim. 2016, 61, 517–524. [Google Scholar]
- Wang Z.; He T.; Qin J.; Wu J.; Li J.; Zi Z.; Liu G.; Wu J.; Sun L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. 10.1016/j.fuel.2015.02.056. [DOI] [Google Scholar]
- Barisano D.; Canneto G.; Nanna F.; Alvino E.; Braccio G.; et al. Steam/oxygen biomass gasification at pilot scale in an internally circulating bubbling fluidized bed reactor. Fuel Process. Technol. 2016, 141, 74–81. 10.1016/j.fuproc.2015.06.008. [DOI] [Google Scholar]
- Mitu M.; Giurcan V.; Razus D.; Oancea D. Inert Gas Influence on Propagation Velocity of Methane-air Laminar Flames. Rev. Chim. 2018, 69, 196–200. 10.37358/RC.18.1.6073. [DOI] [Google Scholar]
- Giurcan V.; Mitu M.; Movileanu C.; Razus D.; Oancea D. Influence of inert additives on small-scale closed vessel explosions of propane-air mixtures. Fire Saf. J. 2020, 111, 102939 10.1016/j.firesaf.2019.102939. [DOI] [Google Scholar]
- Galmiche B.; Halter F.; Foucher F.; Dagaut P. Effects of Dilution on Laminar Burning Velocity of Premixed Methane/Air Flames. Energy Fuels 2011, 25, 948–954. 10.1021/ef101482d. [DOI] [Google Scholar]
- Hu E.; Xue J.; Huang Z.; Iid N. Numerical Study on the Effects of Diluents on the Laminar Burning Velocity of MethaneAir Mixtures. Energy Fuels 2012, 26, 4242–4252. 10.1021/ef300535s. [DOI] [Google Scholar]
- Burbano H. J.; Pareja J.; Amell A. A. Laminar burning velocities and flame stability analysis of H2/CO/air mixtures with dilution of N2 and CO2. Int. J. Hydrogen Energy 2011, 36, 3232–3242. 10.1016/j.ijhydene.2010.11.089. [DOI] [Google Scholar]
- Hu E.; Jin F.; Lun P.; Xue J.; Huang Z.; Yang Z. Experimental and numerical study on the effect of composition on laminar burning velocities of H2/CO/N2/CO2/air mixtures. Int. J. Hydrogen Energy 2012, 37, 18509–18519. 10.1016/j.ijhydene.2012.09.053. [DOI] [Google Scholar]
- Weng W. B.; Wang Z. H.; He Y.; Whiddon R.; Zhou Y. J.; Li Z. S.; Cen K. F. Effect of N2/CO2 dilution on laminar burning velocity of H2–CO–O2 oxy-fuel premixed flame. Int. J. Hydrogen Energy 2015, 40, 1203–1211. 10.1016/j.ijhydene.2014.11.056. [DOI] [Google Scholar]
- Zhang C.; Hu G.; Liao S.; Cheng Q.; Xiang C.; Yuan C. Comparative study on the effects of nitrogen and carbon dioxide on methane/air flames. Energy 2016, 106, 431–442. 10.1016/j.energy.2016.03.087. [DOI] [Google Scholar]
- Mitu M.; Giurcan V.; Razus D.; Oancea D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. 10.1016/j.jhazmat.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Kee R. J.; Rupley F. M.; Miller J. A.. CHEMKIN-III: A FORTRAN Chemical Kinetics Package for the Analysis of Gas-Phase Chemical and Plasma Kinetics, Sandia Report, 1989.
- Wang J.; Huang Z.; Tang C.; Miao H.; Wang X. Numerical study of the effect of hydrogen addition on methane–air mixtures combustion. Int. J. Hydrogen Energy 2009, 34, 1084–1096. 10.1016/j.ijhydene.2008.11.010. [DOI] [Google Scholar]
- Smith G. P.; Golden D. M.; Frenklach M.; Moriarty N. W.; Qin Z.. GRI-Mech 3.0, 1999.
- Wang H.; You X.; Joshi A. V.; Davis S. G.; Laskin A.; Egolfopoulos F.; Law C. K.. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1–C4 Compounds, 2007.
- Chemical-Kinetic Mechanisms for Combustion Applications, San Diego Mechanism Web Page, Mechanical and Aerospace Engineering (Combustion Research); University of California at San Diego, 2014.
- Liao S. Y.; Jiang D. M.; Gao J.; Huang Z. H. Measurements of Markstein Numbers and LaminarBurning Velocities for Natural GasAir Mixtures. Energy Fuels 2004, 18, 316–326. 10.1021/ef034036z. [DOI] [Google Scholar]
- Bradley D.; Gaskell P. H.; Gu X. J. Burning velocities, Markstein lengths, and flame quenching for spherical methane-air flames: a computational study. Combust. Flame 1996, 104, 176–198. 10.1016/0010-2180(95)00115-8. [DOI] [Google Scholar]
- Gu X. J.; Haq M. Z.; Lawes M.; Woolley R. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combust. Flame 2000, 121, 41–58. 10.1016/S0010-2180(99)00142-X. [DOI] [Google Scholar]
- Goswami M. M.; Derks S. S.; Coumans K. K.; Oliveira D. A.; Konnov A. A.; Bastiaans R. R.; Luijten C. C.; Goey D.; et al. The effect of elevated pressures on the laminar burning velocity of methane + air mixtures. Combust. Flame 2013, 160, 1627–1635. 10.1016/j.combustflame.2013.03.032. [DOI] [Google Scholar]
- Pagliaro J. L.; Linteris G. T.; Sunderland P. B.; Baker P. T. Combustion inhibition and enhancement of premixed methane–air flames by halon replacements. Combust. Flame 2015, 162, 41–49. 10.1016/j.combustflame.2014.07.006. [DOI] [Google Scholar]
- Turányi T. Applications of sensitivity analysis to combustion chemistry. Reliab. Eng. Syst. Saf. 1997, 57, 41–48. 10.1016/S0951-8320(97)00016-1. [DOI] [Google Scholar]
- Lutz A. E.; Rupley F. M.; Kee R. J.; Reynolds W. C.; Meeks E.. EQUIL: A CHEMKIN Implementation of STANJAN for Computing Chemical Equilibria; Reaction Design Inc., 1998.
- Liu F.; Guo H.; Smallwood G. J. The chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames. Combust. Flame 2003, 133, 495–497. 10.1016/S0010-2180(03)00019-1. [DOI] [Google Scholar]
- Glassman I.; Yetter R. A.; Glumac N. G.. Combustion; Academic Press, 2014. [Google Scholar]
- Jones A. R. Combustion physics. Phys. Educ. 1985, 20, 292. 10.1088/0031-9120/20/6/006. [DOI] [Google Scholar]
- Hu E.; Xue J.; Huang Z.; Iid N. Numerical Study on the Effects of Diluents on the Laminar Burning Velocity of Methane–Air Mixtures. Energy Fuels 2012, 26, 4242–4252. 10.1021/ef300535s. [DOI] [Google Scholar]
- Galmiche B.; Halter F.; Foucher F.; Dagaut P. Effects of Dilution on Laminar Burning Velocity of Premixed Methane/Air Flames. Energy Fuels 2011, 25, 948–954. 10.1021/ef101482d. [DOI] [Google Scholar]
- Konnov A. A.; Alvarez G. P.; Rybitskaya I. V.; Ruyck J. D. The Effects of Enrichment by Carbon Monoxide on Adiabatic Burning Velocity and Nitric Oxide Formation in Methane Flames. Combust. Sci. Technol. 2008, 181, 117–135. 10.1080/00102200802380173. [DOI] [Google Scholar]
- Lee C. E.; Lee S. R.; Han J. W.; Park J. Numerical study on effect of CO2 addition in flame structure and NOx formation of CH4-air counterflow diffusion flames. Int. J. Energy Res. 2001, 25, 343–354. 10.1002/er.686. [DOI] [Google Scholar]
- Xiang L.; Chu H.; Ren F.; Gu M. Numerical analysis of the effect of CO2 on combustion characteristics of laminar premixed methane/air flames. J. Energy Inst. 2019, 92, 1487–1501. 10.1016/j.joei.2018.06.018. [DOI] [Google Scholar]
- Bozzelli J. W.; Dean A. M. O + NNH: A possible new route for NOX formation in flames. Int. J. Chem. Kinet. 1995, 27, 1097–1109. 10.1002/kin.550271107. [DOI] [Google Scholar]
- Harrington J. E.; Smith G. P.; Berg P. A.; et al. Evidence for a new no production mechanism in flames. Symp. Combust. 1996, 26, 2133–2138. 10.1016/S0082-0784(96)80038-5. [DOI] [Google Scholar]
- Hayhurst A. N.; Hutchinson E. M. Evidence for a New Way of Producing NO via NNH in Fuel-Rich Flames at Atmospheric Pressure. Combust. Flame 1998, 114, 274–279. 10.1016/S0010-2180(97)00328-3. [DOI] [Google Scholar]
- Wang Z.; He T.; Qin J.; Wu J.; Li J.; Zi Z.; Liu G.; Wu J.; Sun L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. 10.1016/j.fuel.2015.02.056. [DOI] [Google Scholar]
- Deng H.; Huang M.; Wen X.; Chen G.; Yao Z.; et al. Numerical investigation of premixed methane-air flame in two-dimensional half open tube in the early stages. Fuel 2020, 272, 117709 10.1016/j.fuel.2020.117709. [DOI] [Google Scholar]
- Deng H.; Yao Z.; Chen G.; Wen X.; Wang F.; Zhang Q.; Dong J.; Huang M. Numerical study of the effects of laminar flame speed on flame dynamics in the early stages of flame propagation in two-dimensional half open tubes. Int. J. Hydrogen Energy 2021, 46, 1288–1301. 10.1016/j.ijhydene.2020.09.226. [DOI] [Google Scholar]
- Yao Z.; Deng H.; Dong J.; Wen X.; Chen G. On explosion characteristics of premixed syngas/air mixtures with different hydrogen volume fractions and ignition positions. Fuel 2020, 288, 119619 10.1016/j.fuel.2020.119619. [DOI] [Google Scholar]
- Yao Z.; Deng H.; Dong J.; Wen X.; Zhang Q.; et al. Effect of the Inclination Angle on Premixed Flame Dynamics in Half-Open Ducts. ACS Omega 2020, 5, 24906–24915. 10.1021/acsomega.0c03667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Chen G.; Zhang A.; Deng H.; Zheng H.; et al. Numerical Simulation of the Effect of CH4/CO Concentration on Combustion Characteristics of Low Calorific Value Syngas. ACS Omega 2021, 6, 5754–5763. 10.1021/acsomega.0c06176. [DOI] [PMC free article] [PubMed] [Google Scholar]